Group 5a On The Periodic Table
Muz Play
Mar 25, 2025 · 5 min read
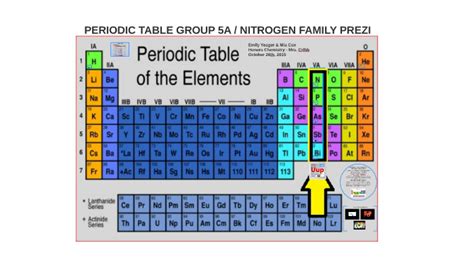
Table of Contents
Group 5A: Delving Deep into the Pnictogens
Group 5A, also known as Group 15 or the pnictogens, represents a fascinating collection of elements exhibiting a diverse range of properties and applications. From the ubiquitous nitrogen in the air we breathe to the crucial role of phosphorus in our DNA, these elements play a vital role in our lives and the world around us. This comprehensive exploration will delve into the characteristics, trends, and applications of each element within Group 5A, highlighting their unique contributions to science and technology.
Understanding the Pnictogens: A Family Portrait
The pnictogens – nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and the synthetic element moscovium (Mc) – share a common electronic configuration: ns²np³. This configuration dictates their chemical behavior, leading to a variety of oxidation states and bonding preferences. However, the significant differences in their atomic radii and electronegativity lead to striking variations in their physical and chemical properties as we move down the group.
Key Trends within Group 5A
-
Atomic Radius: As we descend Group 5A, the atomic radius increases due to the addition of electron shells. This increase influences reactivity and bonding characteristics.
-
Electronegativity: Electronegativity generally decreases down the group. Nitrogen, being highly electronegative, readily forms covalent bonds. Bismuth, conversely, exhibits more metallic character.
-
Ionization Energy: Ionization energy trends generally decrease down the group, reflecting the increasing atomic size and shielding effect.
-
Metallic Character: A gradual shift from non-metallic (nitrogen and phosphorus) to metallic character (antimony and bismuth) is observed as we move down the group. This transition is reflected in their physical properties and reactivity.
Exploring Individual Elements: A Closer Look
Let's examine each element in detail, exploring their unique properties and applications:
Nitrogen (N): The Abundant Gas
Nitrogen, comprising approximately 78% of Earth's atmosphere, is a colorless, odorless, and tasteless gas. Its inertness at standard conditions stems from its strong triple bond (N≡N). However, under specific conditions, nitrogen participates in various reactions:
-
Ammonia Production (Haber-Bosch Process): The synthesis of ammonia (NH₃) from nitrogen and hydrogen is a cornerstone of industrial chemistry, vital for fertilizer production. This process significantly impacts global food production.
-
Nitric Acid Production: The Ostwald process converts ammonia into nitric acid (HNO₃), a key ingredient in fertilizers and explosives.
-
Inert Atmosphere: Nitrogen's inertness makes it suitable for creating inert atmospheres in various industrial processes to prevent oxidation or other unwanted reactions.
-
Biological Significance: Nitrogen is an essential component of amino acids, proteins, and nucleic acids, crucial for all life forms.
Phosphorus (P): A Vital Nutrient and Industrial Workhorse
Phosphorus exists in several allotropic forms, the most common being white phosphorus (highly reactive and toxic), red phosphorus (less reactive), and black phosphorus (the most stable form).
-
Fertilizers: Phosphorus is an essential nutrient for plant growth, making phosphate-containing fertilizers vital for agriculture.
-
DNA and RNA: Phosphorus plays a crucial role in the structure of DNA and RNA, the genetic material of all living organisms.
-
Detergents and Food Additives: Phosphates are used as water softeners in detergents and as food additives.
-
Matches: Red phosphorus is used in the manufacture of safety matches.
Arsenic (As): A Toxic Element with Limited Applications
Arsenic is a metalloid with both metallic and non-metallic properties. It is highly toxic, but it has limited applications:
-
Semiconductors: Arsenic is used in the production of certain semiconductors due to its semiconducting properties.
-
Wood Preservatives: Although its use is now largely restricted due to toxicity concerns, arsenic compounds were historically used in wood preservatives.
-
Medical Applications (Historically): Arsenic compounds were historically used in some medicinal applications, though their toxicity necessitates careful handling and regulation.
Antimony (Sb): A Metalloid with Diverse Uses
Antimony is a brittle, silvery-white metalloid used in various applications:
-
Flame Retardants: Antimony compounds are used as flame retardants in plastics and textiles.
-
Batteries: Antimony is used in lead-acid batteries to enhance their performance.
-
Alloys: Antimony is added to lead alloys to increase their hardness and strength.
Bismuth (Bi): A Low-Toxicity Metal
Bismuth is the heaviest stable pnictogen and exhibits low toxicity compared to other members of the group. Its applications include:
-
Pharmaceuticals: Bismuth compounds are used in various pharmaceuticals, including antacids and medications to treat gastrointestinal disorders.
-
Alloys: Bismuth is used in low-melting alloys, often used in fire sprinklers and solders.
-
Cosmetics: Bismuth oxychloride is used in some cosmetics.
Moscovium (Mc): A Synthetic Element
Moscovium is a synthetic element with extremely short half-life, making its study challenging and limited. Its properties are largely predicted based on its position in the periodic table.
The Pnictogens and the Environment
The environmental impact of pnictogens varies significantly. Excessive phosphorus runoff from fertilizers can lead to eutrophication in water bodies, causing algal blooms and oxygen depletion. Arsenic contamination of groundwater poses a serious health risk in many parts of the world. Responsible use and management of these elements are crucial for environmental protection.
Future Directions and Research
Research on pnictogens continues to evolve, focusing on areas like:
-
Developing new materials: Research into new materials incorporating pnictogens for applications in electronics, energy storage, and catalysis is ongoing.
-
Understanding biological roles: Investigating the biological roles of pnictogens beyond their well-known functions in DNA and proteins is an active area of research.
-
Environmental remediation: Developing strategies to remediate arsenic and phosphorus contamination in soil and water is a critical area of focus.
-
Synthetic element exploration: Research into synthetic pnictogens like moscovium will continue to expand our understanding of the periodic table and the behavior of heavy elements.
Conclusion
Group 5A elements, the pnictogens, represent a fascinating group with a diverse range of properties and applications. From the vital role of nitrogen and phosphorus in biological systems to the industrial uses of arsenic, antimony, and bismuth, these elements are integral to our world. Understanding their properties, trends, and environmental impact is crucial for responsible use and development of new technologies that harness their unique characteristics while minimizing environmental impact. Ongoing research continues to unveil new possibilities and applications for these remarkable elements.
Latest Posts
Latest Posts
-
Definition Of Marketing By Philip Kotler
Mar 28, 2025
-
Formulas For Photosynthesis And Cellular Respiration
Mar 28, 2025
-
The Blank Atom In R 12 Is Believed To Brea Off
Mar 28, 2025
-
Heat Of Neutralization For Hcl And Naoh
Mar 28, 2025
-
Difference Between Selective Media And Differential Media
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Group 5a On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
