Heat Is A Measure Of The Random Of Molecules.
Muz Play
Mar 15, 2025 · 7 min read
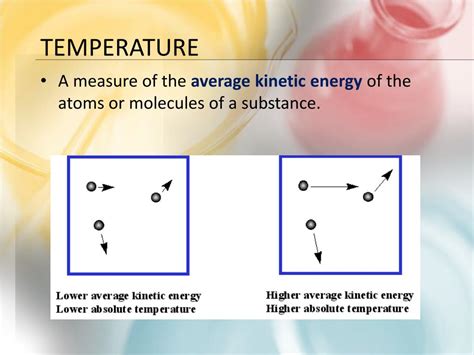
Table of Contents
Heat: A Measure of Molecular Randomness
Heat, a concept fundamental to physics and chemistry, is often misunderstood as a substance itself. However, a deeper understanding reveals heat's true nature: it's a measure of the average kinetic energy of the constituent particles within a system, directly reflecting the randomness of molecular motion. This article delves into the intricate relationship between heat, molecular motion, and the concept of entropy, exploring the microscopic origins of this macroscopic phenomenon.
Understanding Temperature and Kinetic Energy
Before diving into the relationship between heat and molecular randomness, it's crucial to grasp the concept of temperature. Temperature is a measure of the average kinetic energy of the particles in a system. Kinetic energy, in simple terms, is the energy an object possesses due to its motion. For molecules, this means the energy associated with their vibrations, rotations, and translations.
Molecular Motion: The Driving Force Behind Heat
Molecules are in constant motion, even in seemingly stationary objects. This motion, however, is not uniform or directed; it's random. Molecules in a solid vibrate around fixed positions, those in a liquid move more freely, colliding with each other, and those in a gas are in constant, chaotic motion, experiencing frequent collisions and changes in direction.
The speed and intensity of this random molecular motion directly correlate with the temperature and heat content of the system. Higher temperatures imply higher average kinetic energies and more vigorous molecular motion. Conversely, lower temperatures signify slower molecular motion and lower average kinetic energy.
Heat Transfer and the Flow of Kinetic Energy
Heat transfer occurs when there's a difference in temperature between two systems. Heat naturally flows from a hotter system (higher average kinetic energy) to a colder system (lower average kinetic energy), leading to a transfer of kinetic energy. This transfer continues until thermal equilibrium is reached, where both systems achieve the same temperature and the net flow of kinetic energy ceases.
Mechanisms of Heat Transfer: Conduction, Convection, and Radiation
There are three primary mechanisms through which heat transfer occurs:
-
Conduction: Heat transfer through direct contact. When two objects at different temperatures are in contact, kinetic energy is transferred from the hotter object's more energetic molecules to the colder object's less energetic molecules through collisions. This process is particularly efficient in solids where molecules are closely packed.
-
Convection: Heat transfer through the movement of fluids (liquids and gases). As a fluid is heated, its density changes, leading to convection currents. Warmer, less dense fluid rises, while cooler, denser fluid sinks, effectively transporting heat energy.
-
Radiation: Heat transfer through electromagnetic waves. All objects emit electromagnetic radiation, with the intensity and wavelength dependent on their temperature. Hotter objects emit more radiation, transferring heat energy to cooler objects that absorb this radiation. This is how the sun's heat reaches the Earth.
Entropy: The Measure of Disorder
The concept of entropy is intrinsically linked to the randomness of molecular motion and the understanding of heat. Entropy is a thermodynamic property that measures the degree of disorder or randomness in a system. A highly ordered system has low entropy, while a disordered system has high entropy.
The Second Law of Thermodynamics and Entropy Increase
The second law of thermodynamics states that the total entropy of an isolated system can only increase over time or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. This essentially means that natural processes tend to proceed in a direction that increases the overall disorder of the universe.
The relationship between heat and entropy is profound. When heat flows from a hotter object to a colder object, the overall entropy of the system increases. This is because the increased randomness of molecular motion in the colder object (due to the influx of energy) outweighs the decrease in randomness in the hotter object.
Heat Capacity and Specific Heat
Heat capacity and specific heat are crucial concepts in understanding how much heat energy is required to change the temperature of a substance.
-
Heat capacity (C) is the amount of heat required to raise the temperature of a substance by one degree Celsius (or one Kelvin). It's an extensive property, meaning it depends on the mass of the substance.
-
Specific heat (c) is the amount of heat required to raise the temperature of one gram (or one kilogram) of a substance by one degree Celsius (or one Kelvin). It's an intensive property, meaning it's independent of the mass of the substance.
Different substances have different specific heats, reflecting the varying ways their molecules absorb and store kinetic energy. For instance, water has a remarkably high specific heat, meaning it requires a significant amount of heat to raise its temperature. This property is crucial for regulating Earth's climate.
Microscopic View of Heat and Temperature
At the microscopic level, heat manifests as the collective kinetic energy of the molecules within a system. The more energetic the molecules are, the higher the temperature and heat content. This random molecular motion leads to collisions, which transfer energy between molecules, distributing the energy throughout the system.
The distribution of molecular energies follows a statistical distribution, typically a Boltzmann distribution. This distribution shows that not all molecules possess the same energy; there's a range of energies, with some molecules moving faster and others slower. The average of this distribution is directly related to the temperature.
Examples Illustrating the Relationship
Several everyday examples vividly illustrate the connection between heat and molecular randomness:
-
Heating a metal pan: When you heat a metal pan on a stove, the heat energy increases the kinetic energy of the metal atoms, causing them to vibrate more vigorously. This increased vibration is then transferred to the molecules of whatever food is placed in the pan, cooking it.
-
Boiling water: Heating water increases the kinetic energy of water molecules, eventually reaching a point where their average kinetic energy is sufficient to overcome the intermolecular forces holding them together in the liquid phase. This leads to the phase transition from liquid water to water vapor, with molecules moving freely in the gaseous state.
-
Melting ice: Similarly, heating ice increases the kinetic energy of water molecules in their solid state. When this energy surpasses the forces holding them in a rigid lattice, the ice melts into liquid water, characterized by a more random arrangement of molecules.
Advanced Concepts: Statistical Mechanics and Thermodynamics
A more rigorous understanding of heat and its relation to molecular randomness requires delving into statistical mechanics and thermodynamics. These fields use statistical methods to relate microscopic properties (molecular energies and motion) to macroscopic properties (temperature, heat, and entropy).
Statistical mechanics provides a powerful framework for calculating thermodynamic properties from the microscopic behavior of large numbers of particles. It allows for the prediction of macroscopic phenomena from the statistical properties of the underlying microscopic constituents.
Thermodynamics, on the other hand, focuses on the macroscopic properties of systems without necessarily delving into the microscopic details. It deals with concepts such as work, heat, energy, and entropy, providing a general framework for understanding energy transformations and equilibrium states.
Conclusion: Heat as a Manifestation of Randomness
In conclusion, heat is not a substance but a measure of the average kinetic energy of molecules within a system. It is directly tied to the randomness of molecular motion, a concept formalized by the thermodynamic property of entropy. Understanding this fundamental relationship provides a deeper appreciation of heat transfer, temperature, and the behavior of matter at both macroscopic and microscopic levels. The concepts explored here – from basic kinetic theory to advanced statistical mechanics – are crucial in numerous fields, including physics, chemistry, engineering, and materials science. By grasping the essence of heat as a manifestation of molecular randomness, we unlock a deeper understanding of the universe around us.
Latest Posts
Latest Posts
-
The Library Is An Example Of What Type Of Resource
May 09, 2025
-
The Principal Force Driving Movement In Diffusion Is
May 09, 2025
-
What Part Of The Cell Stores Material Within The Cell
May 09, 2025
-
How To Write An Equation For A Function
May 09, 2025
-
Why Does Soap Weaken Hydrogen Bonds
May 09, 2025
Related Post
Thank you for visiting our website which covers about Heat Is A Measure Of The Random Of Molecules. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.