Heat Of Solution Of Ammonium Nitrate
Muz Play
Mar 18, 2025 · 6 min read
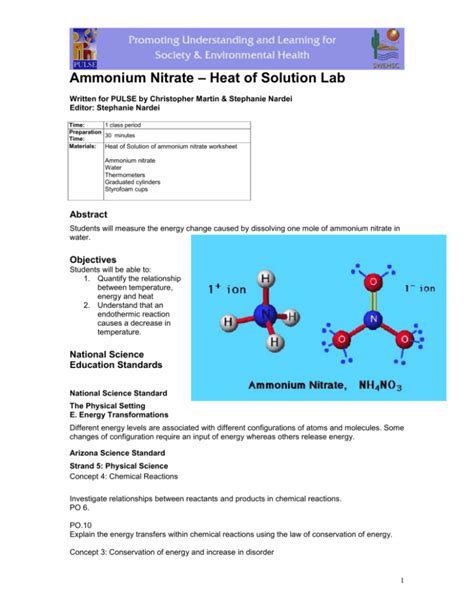
Table of Contents
The Heat of Solution of Ammonium Nitrate: A Deep Dive
Ammonium nitrate (NH₄NO₃), a common chemical compound, exhibits a fascinating property: it absorbs a significant amount of heat when dissolved in water. This process, known as the heat of solution, or enthalpy of solution, is a crucial aspect of its various applications and has significant implications in chemistry and beyond. This comprehensive article delves into the intricacies of ammonium nitrate's heat of solution, exploring its underlying mechanisms, practical applications, and safety considerations.
Understanding Enthalpy of Solution
Before we dive into the specifics of ammonium nitrate, let's establish a foundational understanding of enthalpy of solution. The enthalpy of solution (ΔHsoln) refers to the change in enthalpy that occurs when one mole of a solute dissolves in a large amount of solvent at constant pressure. This enthalpy change represents the net energy absorbed or released during the dissolution process. It's a crucial thermodynamic property, reflecting the interplay between several factors:
-
Lattice Energy: This is the energy required to break apart the ionic bonds in the solid solute. For ammonium nitrate, this involves separating the ammonium (NH₄⁺) and nitrate (NO₃⁻) ions. This process is always endothermic (requires energy).
-
Hydration Energy: This is the energy released when solvent molecules (in this case, water) surround and interact with the dissolved ions. Water molecules, being polar, form strong electrostatic interactions (ion-dipole interactions) with the charged ammonium and nitrate ions. This process is typically exothermic (releases energy).
The overall enthalpy of solution is the sum of these opposing energy changes:
ΔHsoln = ΔHhydration - ΔHlattice
If the hydration energy is greater than the lattice energy, the overall process is exothermic (ΔHsoln < 0), and heat is released. Conversely, if the lattice energy is greater, the process is endothermic (ΔHsoln > 0), and heat is absorbed. Ammonium nitrate's unique behavior falls into the latter category.
The Endothermic Dissolution of Ammonium Nitrate
Unlike many salts that release heat upon dissolving (exothermic dissolution), ammonium nitrate absorbs heat, resulting in a significant temperature drop. This endothermic nature stems from the relatively high lattice energy of ammonium nitrate compared to its hydration energy. While the hydration of the ions does release a substantial amount of energy, it's not enough to offset the energy required to break apart the crystal lattice.
This endothermic behavior is a direct consequence of the strong ionic bonds within the ammonium nitrate crystal structure. Breaking these bonds requires a considerable input of energy. Furthermore, the relatively weaker ion-dipole interactions between the ammonium and nitrate ions and water molecules contribute to the overall endothermic nature of the dissolution. The energy needed to disrupt the crystal structure is simply larger than the energy gained from the hydration process.
Factors Influencing the Heat of Solution
Several factors can subtly influence the measured heat of solution of ammonium nitrate:
-
Concentration: The heat of solution can vary slightly depending on the concentration of the ammonium nitrate solution. At very high concentrations, interionic interactions can become more significant, affecting the overall enthalpy change.
-
Temperature: The temperature of the water also plays a role. The heat capacity of water changes with temperature, which can influence the observed temperature change during dissolution.
-
Purity of Ammonium Nitrate: Impurities in the ammonium nitrate sample can affect the heat of solution. Impurities can alter the lattice energy and hydration interactions.
Practical Applications Leveraging the Endothermic Nature
The unique endothermic nature of ammonium nitrate's dissolution has led to a wide array of practical applications:
-
Instant Cold Packs: This is perhaps the most well-known application. Ammonium nitrate is frequently used in commercially available instant cold packs. These packs typically contain a sealed pouch of ammonium nitrate crystals and a separate pouch of water. Upon breaking the inner pouch, the ammonium nitrate dissolves in the water, absorbing heat from the surroundings and creating a cooling effect. This is invaluable for treating minor injuries, reducing swelling, and providing temporary relief from pain.
-
Fertilizers: Ammonium nitrate's primary use is as a nitrogen-rich fertilizer. While the endothermic nature of its dissolution isn't directly related to its fertilizing properties, the cooling effect can be beneficial in hot climates, preventing potential damage to plant roots.
-
Industrial Cooling Applications: In some industrial processes, the endothermic dissolution of ammonium nitrate can be utilized for localized cooling. This is particularly useful in applications where precise temperature control is crucial.
Safety Considerations and Handling Ammonium Nitrate
While ammonium nitrate has numerous beneficial applications, it's essential to handle it with caution due to its potential hazards:
-
Oxidizer: Ammonium nitrate is a strong oxidizer and can react violently with reducing agents, potentially leading to explosions or fires. It should never be mixed with combustible materials.
-
Environmental Concerns: Improper disposal of ammonium nitrate can have adverse environmental consequences, potentially contaminating water sources and harming aquatic life. Responsible disposal methods should always be followed.
-
Health Hazards: Inhalation of ammonium nitrate dust can irritate the respiratory system. Skin contact can also cause irritation. Appropriate personal protective equipment (PPE) should be used when handling ammonium nitrate.
-
Explosive Potential: Under specific conditions, such as confinement and the presence of a strong ignition source, ammonium nitrate can detonate. This underscores the importance of safe storage and handling practices.
The specific hazards associated with ammonium nitrate depend on the form (e.g., crystals, prills, solutions), the quantity, and the presence of other substances. Proper training and adherence to safety protocols are crucial for preventing accidents.
Further Research and Advanced Topics
The heat of solution of ammonium nitrate is a complex phenomenon influenced by numerous factors. Further research could explore:
-
Molecular Dynamics Simulations: Computational methods such as molecular dynamics simulations can provide detailed insights into the interactions between ammonium and nitrate ions and water molecules during the dissolution process. This can help refine our understanding of the energy changes involved.
-
Effect of Different Solvents: Investigating the heat of solution of ammonium nitrate in solvents other than water could reveal valuable information about the role of solvent properties in the dissolution process.
-
Influence of Pressure: Studying the effect of pressure on the heat of solution can offer further insights into the thermodynamics of the system.
Conclusion: A Versatile Compound with Unique Properties
Ammonium nitrate, with its unique endothermic heat of solution, is a versatile chemical compound with applications ranging from everyday cold packs to industrial processes. Understanding its thermodynamic properties, including its enthalpy of solution, is vital for its safe and effective use. Proper handling and disposal practices are crucial to mitigate potential hazards associated with its oxidizing and explosive properties. Continued research into its behavior under various conditions will undoubtedly lead to even more advanced applications and a deeper understanding of its fascinating chemistry. The simple act of dissolving ammonium nitrate in water provides a compelling example of the intricate energy changes that govern chemical reactions and their practical implications in our lives.
Latest Posts
Latest Posts
-
What Is The Percentage Of Truth In A Joke
Mar 18, 2025
-
T Test Formula For Dependent Samples
Mar 18, 2025
-
Eriksons Stage Of Integrity Vs Despair
Mar 18, 2025
-
What Is The Difference Between Microscopic And Macroscopic
Mar 18, 2025
-
Determine The Degrees Of Freedom For The F Statistic
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Heat Of Solution Of Ammonium Nitrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
