Horizontal Row On The Periodic Table
Muz Play
Mar 27, 2025 · 6 min read
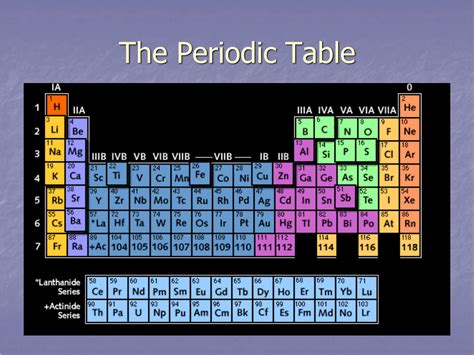
Table of Contents
Unveiling the Secrets of the Periodic Table's Horizontal Rows: A Deep Dive into Periods
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While the vertical columns, or groups, showcase elements with similar characteristics, the horizontal rows, known as periods, tell a different story – the story of electron shell filling and the gradual evolution of elemental properties across a row. This comprehensive exploration delves into the intricacies of periods, examining their structure, trends, and significance in understanding chemical behavior.
Understanding the Structure of Periods
Each period on the periodic table corresponds to a principal energy level (shell) that is being filled with electrons. The first period, the shortest, only has two elements: hydrogen (H) and helium (He). This is because the first energy level (n=1) can only accommodate a maximum of two electrons. As we move down the table to subsequent periods, the number of elements increases because more electrons can be accommodated in higher energy levels. The second period (n=2) holds eight elements, while the third period (n=3) also contains eight. However, from the fourth period onward, the pattern becomes more complex due to the filling of d and f subshells.
The Significance of Electron Configuration
The arrangement of electrons within an atom's energy levels, its electron configuration, directly dictates its chemical properties. Elements within the same period share the same highest principal quantum number (n), meaning their outermost electrons occupy the same energy level. This shared characteristic significantly impacts their reactivity and bonding behavior. Understanding electron configuration is paramount to predicting how elements within a period will interact.
Periodicity of Properties: A Gradual Shift
As we traverse a period from left to right, we witness a systematic change in the properties of elements. This is due to the sequential addition of protons and electrons, leading to increased nuclear charge and a gradual decrease in atomic radius. These changes are reflected in the following key periodic trends:
-
Atomic Radius: Atomic radius generally decreases across a period. This is primarily because the increasing nuclear charge pulls the electrons closer to the nucleus, counteracting the addition of electrons to the same energy level.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The stronger attraction of the nucleus to the electrons makes it more difficult to remove them.
-
Electron Affinity: Electron affinity, the energy change when an atom gains an electron, generally increases across a period (with some exceptions). The increased nuclear charge makes the atom more likely to attract an additional electron.
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. This is a direct consequence of the increasing nuclear charge.
-
Metallic Character: Metallic character generally decreases across a period. Elements on the left side of a period tend to be more metallic (easily lose electrons), while those on the right are more non-metallic (tend to gain electrons).
Period-by-Period Analysis: A Detailed Examination
Let's examine the characteristics of each period in more detail:
Period 1: The Pioneers
This period, the shortest, features hydrogen and helium. Hydrogen, a highly reactive nonmetal, is unique due to its single electron and its ability to form both covalent and ionic bonds. Helium, a noble gas, is exceptionally unreactive due to its full electron shell.
Period 2: The Lightweights
Period 2 contains lithium (Li) to neon (Ne), showcasing a dramatic range of properties. Lithium, an alkali metal, is highly reactive. Beryllium (Be), an alkaline earth metal, is less reactive. Boron (B), the first metalloid, exhibits properties of both metals and nonmetals. Carbon (C), nitrogen (N), oxygen (O), and fluorine (F) are nonmetals with increasing electronegativity. Neon (Ne), a noble gas, is inert.
Period 3: The Mirror Image
Period 3 mirrors many trends seen in Period 2, with sodium (Na) to argon (Ar). Sodium is highly reactive like lithium, while chlorine (Cl) is a highly reactive nonmetal similar to fluorine. The metalloids silicon (Si) and phosphorus (P) demonstrate properties intermediate between metals and nonmetals. Argon (Ar), like neon, is a noble gas.
Period 4 and Beyond: The Complexity Increases
Starting from Period 4, the complexity increases with the introduction of the d-block elements (transition metals). The filling of the d orbitals leads to a less pronounced trend in properties across the period. The gradual change in properties is less distinct than in the previous periods due to the shielding effect of inner electrons. Similar complexities arise in subsequent periods with the filling of the f-block elements (lanthanides and actinides).
The Significance of Periods in Chemical Reactions
The period an element belongs to significantly influences its chemical behavior. For example, elements in the same period may participate in reactions with varying degrees of reactivity, influenced by their electronegativity and ionization energy. Understanding the periodic trends within a period helps predict the products and the course of chemical reactions.
Predicting Reactivity: A Powerful Tool
By examining the position of elements within a period, we can predict their relative reactivity in chemical reactions. Alkali metals (Group 1) will readily react with halogens (Group 17) to form ionic compounds. The reactivity of both alkali metals and halogens increases as we move down their respective groups.
Applications and Significance
Understanding the trends and variations within periods is crucial in several fields:
-
Material Science: The properties of elements within a period are crucial for developing new materials with desired characteristics, such as conductivity, strength, and reactivity.
-
Catalysis: The ability of transition metals to exhibit multiple oxidation states is a key factor in their catalytic activity, important in industrial processes and environmental remediation.
-
Biological Systems: The properties of elements within specific periods play essential roles in biological processes, affecting enzyme activity, structural integrity, and electron transport.
-
Pharmaceutical Development: The understanding of period trends helps in designing and synthesizing new drugs with improved efficacy and reduced side effects.
Conclusion: A Journey Across the Horizontal Landscape
The horizontal rows of the periodic table, the periods, represent a fascinating journey across the evolution of elemental properties. From the simple arrangement of the first two periods to the complexities introduced by the d- and f-block elements, the periods offer profound insights into the underlying principles governing the behavior of matter. By understanding the systematic trends in atomic radius, ionization energy, electronegativity, and other properties, we can predict and interpret the chemical behavior of elements, paving the way for advancements across numerous scientific and technological domains. The exploration of periods continues to be a vibrant area of research, promising to further unveil the rich tapestry of chemistry and its boundless applications. This deep dive into the horizontal rows of the periodic table underscores their pivotal role in our understanding of the chemical world. Further investigation into specific periods and the nuanced interactions between elements will continue to enrich our knowledge and facilitate groundbreaking discoveries.
Latest Posts
Latest Posts
-
Where Does Transcription Take Place In The Eukaryotic Cell
Mar 30, 2025
-
Difference Between Simple Distillation And Fractional
Mar 30, 2025
-
In A Nonpolar Covalent Bond Electrons Are
Mar 30, 2025
-
Ion Channels That Are Always Open Are Called
Mar 30, 2025
-
What Directly Causes The Athenians To Hide In Their Homes
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Row On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
