How Do Intermolecular Forces Affect Viscosity
Muz Play
Apr 06, 2025 · 6 min read
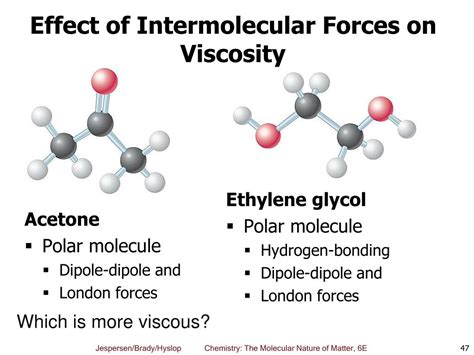
Table of Contents
How Do Intermolecular Forces Affect Viscosity?
Viscosity, the resistance of a fluid to flow, is a crucial property impacting various applications, from engine lubrication to blood circulation. Understanding the factors governing viscosity is essential for controlling and predicting fluid behavior. One of the most significant influences on viscosity is the strength of intermolecular forces (IMFs) present within the fluid. This article delves deep into the intricate relationship between intermolecular forces and viscosity, exploring the different types of IMFs and their respective effects.
Understanding Viscosity and Intermolecular Forces
Viscosity is a measure of a fluid's internal resistance to flow. A highly viscous fluid, like honey, flows slowly, while a low-viscosity fluid, like water, flows readily. This resistance arises from the interactions between the fluid's constituent molecules. Stronger interactions translate to greater resistance to flow, thus higher viscosity.
Intermolecular forces, as the name suggests, are the attractive or repulsive forces that act between molecules. They are weaker than the intramolecular forces (bonds within a molecule) but play a crucial role in determining the physical properties of substances, including viscosity. Several types of IMFs exist, each with varying strengths:
-
London Dispersion Forces (LDFs): These are the weakest type of IMF and are present in all molecules, regardless of their polarity. They arise from temporary, instantaneous fluctuations in electron distribution around a molecule, creating temporary dipoles that induce dipoles in neighboring molecules. Larger molecules with more electrons generally exhibit stronger LDFs.
-
Dipole-Dipole Forces: These forces occur between polar molecules, which have a permanent dipole moment due to an uneven distribution of electrons. The positive end of one molecule attracts the negative end of another, leading to a stronger attraction than LDFs.
-
Hydrogen Bonding: This is a special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to a lone pair of electrons on another electronegative atom. Hydrogen bonds are significantly stronger than other dipole-dipole forces.
-
Ion-Dipole Forces: These interactions occur between ions (charged particles) and polar molecules. The charged ion attracts the oppositely charged end of the polar molecule. These are stronger than dipole-dipole forces.
The Influence of Intermolecular Forces on Viscosity
The strength of IMFs directly impacts a fluid's viscosity. The stronger the IMFs, the greater the resistance to flow and, consequently, the higher the viscosity. This is because stronger attractive forces hold the molecules more tightly together, making it more difficult for them to move past each other.
Liquids with Strong Intermolecular Forces: High Viscosity
Liquids with strong hydrogen bonds, such as water and glycerol, tend to have high viscosities. The extensive hydrogen bonding network creates a significant resistance to flow. Glycerol, with its multiple hydroxyl (-OH) groups, forms a complex web of hydrogen bonds, resulting in its exceptionally high viscosity. Similarly, the strong hydrogen bonding in water contributes to its relatively higher viscosity compared to other liquids with weaker IMFs.
Liquids with Weak Intermolecular Forces: Low Viscosity
Liquids with weaker IMFs, such as nonpolar hydrocarbons (like pentane or hexane), exhibit low viscosities. Their molecules interact primarily through weak LDFs, allowing them to move more freely past each other. The relatively small size of these molecules also contributes to their low viscosity. The absence of strong hydrogen bonding or dipole-dipole interactions further minimizes intermolecular resistance to flow.
The Role of Molecular Size and Shape
Besides the type of IMF, the size and shape of the molecules also affect viscosity. Larger molecules have more electrons, leading to stronger LDFs and increased viscosity. Furthermore, the shape of molecules plays a crucial role. Long, chain-like molecules tend to entangle more easily, increasing resistance to flow and enhancing viscosity. This is why polymers, with their long, intertwined chains, exhibit high viscosities. Conversely, smaller, more spherical molecules experience less entanglement, resulting in lower viscosity.
Temperature's Impact on Viscosity and Intermolecular Forces
Temperature significantly affects viscosity. As temperature increases, the kinetic energy of the molecules increases. This increased kinetic energy overcomes the attractive intermolecular forces, allowing molecules to move more freely past one another. Consequently, viscosity decreases with increasing temperature. This is why honey flows more easily when it's warm compared to when it's cold. The higher temperature weakens the hydrogen bonds and other IMFs, reducing the resistance to flow.
Conversely, decreasing the temperature reduces the kinetic energy of the molecules. This allows the intermolecular forces to dominate, restricting molecular movement and increasing viscosity. At sufficiently low temperatures, many liquids may even solidify as the intermolecular forces completely overwhelm the kinetic energy, leading to a rigid structure.
Viscosity and Applications: Real-World Examples
The relationship between viscosity and intermolecular forces is crucial in numerous applications:
-
Lubricants: Engine oils rely on their viscosity to effectively reduce friction between moving parts. The viscosity of these oils is carefully engineered to remain within a specific range across different temperatures, ensuring optimal lubrication. The presence of long-chain hydrocarbons and additives influences their viscosity.
-
Blood Flow: The viscosity of blood plays a vital role in its circulation. Changes in blood viscosity, often caused by changes in red blood cell concentration or protein levels, can affect blood flow and cardiovascular health.
-
Food Processing: The viscosity of various food products, such as sauces, jams, and syrups, is critical for their texture and palatability. Manufacturers control viscosity by adjusting the composition and processing methods to achieve the desired consistency.
-
Polymer Processing: The viscosity of polymer melts is crucial in determining their processing characteristics. The ability to control viscosity allows for the shaping of polymers into various forms through extrusion, injection molding, and other processes.
-
Pharmaceutical Industry: The viscosity of many pharmaceutical formulations is important for their stability, delivery methods, and bioavailability. Proper viscosity control ensures the consistent dosage and effective delivery of medications.
Advanced Concepts and Further Considerations
While the relationship between intermolecular forces and viscosity is relatively straightforward, several advanced concepts and considerations add complexity:
-
Non-Newtonian Fluids: Some fluids exhibit non-Newtonian behavior, meaning their viscosity changes with the applied shear stress or shear rate. These fluids don't follow the simple relationship between viscosity and temperature discussed above. Examples include ketchup, paints, and some biological fluids.
-
Molecular Weight Distribution: In polymers, the distribution of molecular weights significantly influences viscosity. A broader distribution leads to greater entanglement and higher viscosity.
-
Solvent Effects: For solutions, the solvent's properties significantly influence the overall viscosity. The solvent's IMF interactions with the solute molecules can either increase or decrease the overall viscosity.
-
Pressure Effects: Pressure can also affect viscosity, especially at high pressures, though the effect is generally less pronounced than that of temperature.
Conclusion: A Comprehensive View
The viscosity of a fluid is intrinsically linked to the strength of its intermolecular forces. Stronger IMFs, such as hydrogen bonds and ion-dipole forces, lead to higher viscosities, while weaker forces, like LDFs, result in lower viscosities. Factors like molecular size, shape, temperature, and pressure also play important roles in determining a fluid's viscosity. Understanding this intricate interplay is critical in various fields, from engineering and materials science to biology and medicine. Further research into the complexities of non-Newtonian fluids and the influence of other factors will continue to refine our understanding of this fundamental property of fluids. This knowledge is vital for optimizing processes and developing new materials with tailored viscosities to meet specific needs.
Latest Posts
Latest Posts
-
Was Adolf Hitler A Great Leader
Apr 07, 2025
-
How Is Energy Used In A Cell
Apr 07, 2025
-
Ground State Orbital Diagrams And Electron Configurations
Apr 07, 2025
-
The Oldest Religion In Southwest Asia Is
Apr 07, 2025
-
An Energy Storage Polysaccharide In Plants Is Called
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Do Intermolecular Forces Affect Viscosity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
