How Do Positive Ions And Negative Ions Form
Muz Play
Apr 02, 2025 · 5 min read
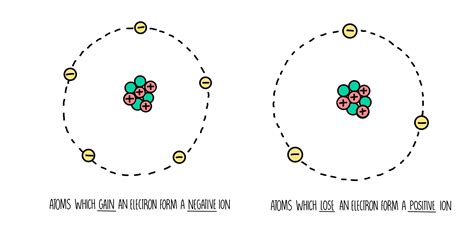
Table of Contents
- How Do Positive Ions And Negative Ions Form
- Table of Contents
- How Do Positive and Negative Ions Form? Understanding the Basics of Ionization
- The Fundamentals: Atoms and Their Structure
- The Birth of Ions: The Ionization Process
- Mechanisms of Ion Formation: A Closer Look
- 1. Electron Transfer in Chemical Reactions
- 2. Ionization by Radiation: The Power of Electromagnetic Waves
- 3. Ionization by Collision: High-Energy Impacts
- 4. Ionization by Electron Impact: Adding Energy Directly
- The Role of Ionization in Everyday Life and Beyond
- 1. Atmospheric Chemistry and Weather:
- 2. Combustion and Flames:
- 3. Biological Systems:
- 4. Medical Applications:
- 5. Industrial Processes:
- The Significance of Ion Stability
- Conclusion: A World of Charged Particles
- Latest Posts
- Latest Posts
- Related Post
How Do Positive and Negative Ions Form? Understanding the Basics of Ionization
The world around us is teeming with electrically charged particles called ions. These ions, fundamental building blocks in chemistry and physics, play crucial roles in everything from the conductivity of electricity to the processes occurring within our bodies. Understanding how these ions – specifically positive and negative ions – form is crucial to grasping many natural phenomena and technological applications. This article delves into the fascinating process of ionization, explaining the mechanisms that lead to the creation of positive and negative ions.
The Fundamentals: Atoms and Their Structure
Before we dive into ion formation, let's briefly review the structure of an atom. An atom is the basic unit of matter and consists of a central nucleus containing positively charged protons and neutral neutrons. Surrounding the nucleus are negatively charged electrons, orbiting in specific energy levels or shells. The number of protons in the nucleus determines the element's atomic number and its identity. Crucially, for a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
Key takeaway: A neutral atom has an equal number of protons and electrons.
The Birth of Ions: The Ionization Process
Ionization is the process by which a neutral atom gains or loses electrons, resulting in a net electrical charge. This charge imbalance transforms the neutral atom into an ion. If an atom loses one or more electrons, it becomes positively charged, forming a cation. Conversely, if an atom gains one or more electrons, it becomes negatively charged, forming an anion.
Key takeaway: Ionization involves the gain or loss of electrons, creating charged particles (ions).
Mechanisms of Ion Formation: A Closer Look
Several mechanisms can lead to ionization. Let's explore some of the most prominent ones:
1. Electron Transfer in Chemical Reactions
One of the most common ways ions form is through electron transfer during chemical reactions. This often occurs between atoms with vastly different electronegativities. Electronegativity measures an atom's tendency to attract electrons. When a highly electronegative atom interacts with a less electronegative atom, the electronegative atom can "steal" one or more electrons from the less electronegative atom.
For instance, consider the reaction between sodium (Na) and chlorine (Cl). Sodium has a low electronegativity and readily loses one electron to achieve a stable electron configuration (octet rule). Chlorine, with high electronegativity, readily accepts this electron to also achieve a stable configuration. This electron transfer creates a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl−), forming the ionic compound sodium chloride (NaCl), commonly known as table salt.
Example: Formation of Sodium Chloride (NaCl) - a classic example of ionic bonding driven by electron transfer.
2. Ionization by Radiation: The Power of Electromagnetic Waves
Electromagnetic radiation, such as ultraviolet (UV) light, X-rays, and gamma rays, possesses sufficient energy to knock electrons out of atoms. This process, known as photoionization, generates positively charged ions and free electrons. The energy of the radiation must exceed the atom's ionization energy – the minimum energy required to remove an electron.
Example: The ozone layer protects us from harmful UV radiation that could ionize molecules in our atmosphere and within our bodies.
3. Ionization by Collision: High-Energy Impacts
High-energy collisions between atoms or molecules can also cause ionization. This often occurs in high-temperature environments like plasma or during particle collisions in accelerators. The kinetic energy from the collision can transfer enough energy to an atom to eject an electron, creating an ion.
Example: Lightning strikes – the intense energy causes ionization of air molecules.
4. Ionization by Electron Impact: Adding Energy Directly
A free electron with sufficient kinetic energy can collide with a neutral atom and transfer enough energy to remove an electron, producing a positive ion and two free electrons. This mechanism is commonly used in various technologies, such as mass spectrometry and gas discharge lamps.
Example: Gas discharge lamps - a common application of ionization via electron impact.
The Role of Ionization in Everyday Life and Beyond
Ionization isn't just a laboratory phenomenon; it plays a critical role in various natural processes and technological applications:
1. Atmospheric Chemistry and Weather:
Ions are abundant in the atmosphere, formed by cosmic rays, ultraviolet radiation, and lightning. These atmospheric ions influence cloud formation, precipitation patterns, and even air quality.
2. Combustion and Flames:
Combustion processes involve ionization, creating charged particles that contribute to the flame's conductivity and its characteristic light emission.
3. Biological Systems:
Ions are essential for life. Sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions play crucial roles in nerve impulse transmission, muscle contraction, and maintaining osmotic balance in our cells.
4. Medical Applications:
Ionizing radiation is used in medical imaging techniques like X-rays and radiotherapy to treat cancer. Mass spectrometry, a technique reliant on ion detection, is used extensively in medical diagnostics.
5. Industrial Processes:
Ionization is utilized in various industrial processes, including:
- Electroplating: Uses ionic solutions to deposit metal coatings onto surfaces.
- Semiconductor manufacturing: Ion implantation is crucial for doping semiconductors and controlling their electrical properties.
- Air purification: Ionizers are used to remove pollutants from the air by charging them and then collecting them on a filter.
The Significance of Ion Stability
The stability of ions is crucial. Atoms tend to lose or gain electrons to achieve a stable electron configuration, often involving a full outer electron shell (octet rule). This stability significantly influences the chemical reactivity and behavior of ions. Highly reactive ions readily participate in chemical reactions to achieve stability, while more stable ions are less prone to reactions.
Conclusion: A World of Charged Particles
The formation of positive and negative ions through ionization processes is a fundamental concept in chemistry and physics. The mechanisms involved—electron transfer, radiation, collisions, and electron impact—determine the types and abundance of ions in various environments. These ions play critical roles in atmospheric chemistry, biological processes, technological applications, and many other aspects of our world, highlighting their importance in shaping our understanding of the natural world and driving advancements in technology. From the simplest chemical reaction to the complex workings of our bodies and the vastness of space, ions are fundamental to the world we live in. Continued research into ionization processes holds the key to further advancements in diverse scientific fields and technological innovations.
Latest Posts
Latest Posts
-
Abiotic Factors In A Marine Biome
Apr 09, 2025
-
How To Interpret A Karyotype Answer Key
Apr 09, 2025
-
The Form Acidic Compounds With Hydrogen
Apr 09, 2025
-
What Is The Purpose Of Age Structures
Apr 09, 2025
-
What Is The Difference Between Glycogen And Starch
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How Do Positive Ions And Negative Ions Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.