How Do You Calculate The Solubility Of A Substance
Muz Play
Mar 17, 2025 · 6 min read
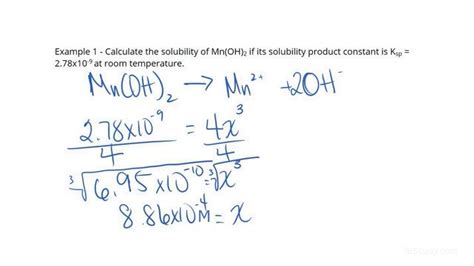
Table of Contents
How Do You Calculate the Solubility of a Substance?
Solubility, a fundamental concept in chemistry and various related fields, quantifies the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Understanding how to calculate solubility is crucial in numerous applications, ranging from pharmaceutical drug development to environmental remediation. This comprehensive guide explores the diverse methods and factors influencing solubility calculations.
Understanding Solubility: A Foundation
Before delving into calculation methods, let's clarify the core concepts. Solubility is typically expressed as the concentration of a saturated solution. A saturated solution contains the maximum amount of solute that can dissolve under given conditions. Any additional solute added will remain undissolved. Several ways exist to express solubility:
- Molar Solubility: The number of moles of solute dissolved per liter of saturated solution (mol/L or M). This is a common and convenient unit for calculations.
- Mass Solubility: The mass of solute dissolved per unit volume of saturated solution (e.g., g/L, mg/mL). This is often used in practical applications where mass measurements are more readily available.
- Weight Percentage: The mass of solute dissolved per 100 grams of solution (%). Useful for representing the overall composition of a solution.
- Mole Fraction: The ratio of the moles of solute to the total moles of solute and solvent. This is particularly useful in thermodynamic calculations.
Factors Affecting Solubility
Several factors significantly impact a substance's solubility, influencing the accuracy of any calculation. These factors must be considered when determining or predicting solubility:
1. Temperature:
Temperature's effect on solubility varies depending on whether the dissolution process is endothermic (absorbs heat) or exothermic (releases heat). Generally, increasing the temperature increases the solubility of solids in liquids for endothermic processes, as heat input favors dissolution. Conversely, the solubility of gases in liquids usually decreases with increasing temperature because gas dissolution is often exothermic.
2. Pressure:
Pressure primarily affects the solubility of gases in liquids. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of the gas above the solution. Increasing the pressure increases the solubility of the gas. The effect of pressure on the solubility of solids and liquids is usually negligible.
3. Nature of the Solute and Solvent:
The "like dissolves like" principle is fundamental. Polar solvents (e.g., water) tend to dissolve polar solutes (e.g., salts, sugars), while nonpolar solvents (e.g., hexane) dissolve nonpolar solutes (e.g., fats, oils). The intermolecular forces between solute and solvent molecules play a critical role in determining solubility. Stronger attractive forces lead to higher solubility.
4. Common Ion Effect:
The presence of a common ion in the solution decreases the solubility of a sparingly soluble ionic compound. This is due to the shift in equilibrium according to Le Chatelier's principle.
5. pH:
The pH of the solution can significantly affect the solubility of weak acids and bases. Changing the pH alters the ionization state of the solute, influencing its solubility.
Methods for Calculating Solubility
Calculating solubility can involve various approaches, ranging from empirical measurements to theoretical predictions based on thermodynamic principles.
1. Experimental Determination:
The most direct method is to experimentally determine solubility. This involves saturating a solvent with a solute at a specific temperature and pressure, then analyzing the saturated solution to determine the solute concentration. Techniques like titration, spectrophotometry, or chromatography can be used for this analysis. While accurate, this method is time-consuming and resource-intensive.
2. Using Solubility Product Constant (Ksp):
For sparingly soluble ionic compounds, solubility can be calculated using the solubility product constant (Ksp). Ksp is the equilibrium constant for the dissolution of a sparingly soluble ionic compound in water. The Ksp value is temperature-dependent. For example, consider the dissolution of silver chloride (AgCl):
AgCl(s) <=> Ag+(aq) + Cl-(aq)
Ksp = [Ag+][Cl-]
If the molar solubility of AgCl is 's', then [Ag+] = [Cl-] = s. Therefore, Ksp = s². Knowing the Ksp value, we can calculate 's', which represents the molar solubility.
3. Using Activity Coefficients:
In concentrated solutions, the interaction between ions becomes significant, deviating from ideal behavior. Activity coefficients correct for these deviations. The activity (a) of an ion is related to its concentration (c) by the activity coefficient (γ):
a = γc
Using activity coefficients in solubility calculations leads to more accurate results for concentrated solutions.
4. Thermodynamic Calculations:
Sophisticated thermodynamic models can predict solubility based on the Gibbs free energy of dissolution. These models incorporate parameters like enthalpy and entropy changes during dissolution, providing a more fundamental understanding of the process. However, these calculations often require extensive data and complex computational methods.
5. Predictive Software and Databases:
Several software packages and databases provide solubility predictions based on various models and experimental data. These tools can be valuable for estimating solubility when experimental data are limited or unavailable.
Applications of Solubility Calculations
Understanding and calculating solubility is critical in various fields:
-
Pharmaceutical Industry: Solubility is crucial for drug formulation. Drugs must possess adequate solubility to be absorbed effectively into the bloodstream. Solubility calculations help in designing drug delivery systems and optimizing bioavailability.
-
Environmental Science: Solubility plays a vital role in assessing the environmental fate of pollutants. The solubility of contaminants determines their mobility and bioavailability in soil and water systems. Solubility calculations are used in environmental risk assessment and remediation strategies.
-
Chemical Engineering: Solubility calculations are essential in designing separation processes like crystallization, precipitation, and extraction. Understanding solubility helps optimize process efficiency and product purity.
-
Food Science: Solubility affects the texture, taste, and stability of food products. Solubility calculations are used in formulating food ingredients and preserving food quality.
-
Geochemistry: Solubility is important in understanding mineral formation and dissolution in geological systems. Solubility calculations help in interpreting geochemical data and predicting mineral behavior in different environments.
Advanced Considerations and Challenges
While the methods described above provide a framework for solubility calculations, several complexities and challenges remain:
-
Polymorphism: Many substances exist in multiple crystalline forms (polymorphs), each with a different solubility. Identifying the polymorph present is crucial for accurate solubility calculations.
-
Solid Solutions: The solubility of a component in a solid solution depends on the composition and properties of the entire solid mixture. Calculations become more complex than those for simple dissolution in a pure solvent.
-
Non-ideal Solutions: Deviations from ideal behavior can significantly impact solubility calculations, particularly in concentrated solutions or when strong intermolecular interactions are present. Advanced models are needed to accurately represent these systems.
-
Kinetic Limitations: Although thermodynamic calculations predict the equilibrium solubility, the rate at which dissolution occurs can be slow due to kinetic limitations like slow diffusion or surface reactions.
Conclusion
Calculating the solubility of a substance is a multifaceted process encompassing experimental methods, theoretical models, and practical applications. The choice of method depends on the specific substance, the desired accuracy, and the available resources. While relatively straightforward for simple cases, challenges arise with complex systems, requiring advanced computational techniques and a thorough understanding of the underlying physical chemistry. Regardless of the chosen approach, accurate solubility calculations are crucial for numerous scientific and technological advancements. The information provided here forms a solid foundation for understanding this essential concept and its diverse applications. Further research into specialized methodologies and software will enhance one's ability to handle a broader range of solubility challenges.
Latest Posts
Latest Posts
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Do You Calculate The Solubility Of A Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
