How Do You Know If A Reaction Is Redox
Muz Play
Mar 26, 2025 · 6 min read
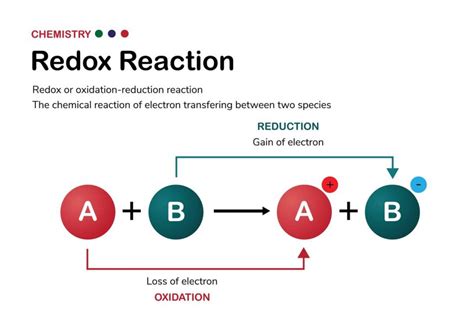
Table of Contents
How Do You Know if a Reaction is Redox? A Comprehensive Guide
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin countless natural and industrial phenomena. Understanding how to identify a redox reaction is crucial for anyone studying chemistry, from high school students to advanced researchers. This comprehensive guide will equip you with the knowledge and tools to confidently determine whether a given chemical reaction involves the transfer of electrons – the hallmark of a redox reaction.
Understanding the Fundamentals of Redox Reactions
Before diving into identification methods, let's solidify our understanding of the core principles. Redox reactions are characterized by the simultaneous occurrence of two half-reactions:
-
Oxidation: The loss of electrons by an atom, ion, or molecule. The species undergoing oxidation is called the reducing agent because it causes the reduction of another species. Its oxidation state increases.
-
Reduction: The gain of electrons by an atom, ion, or molecule. The species undergoing reduction is called the oxidizing agent because it causes the oxidation of another species. Its oxidation state decreases.
The key takeaway: electrons are always transferred in a redox reaction. One species loses electrons (oxidation), and another species gains those same electrons (reduction). These processes are inseparable; they always happen together.
Method 1: Identifying Redox Reactions Using Oxidation States
This is arguably the most common and reliable method for identifying redox reactions. It involves assigning oxidation states (oxidation numbers) to each atom in the reactants and products and then observing changes in these numbers.
Assigning Oxidation States: A Step-by-Step Guide
Assigning oxidation states might seem daunting at first, but with practice, it becomes second nature. Here are some crucial rules to remember:
-
The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of Na in Na(s) is 0, and the oxidation state of Cl in Cl₂(g) is 0.
-
The oxidation state of a monatomic ion is equal to its charge. For example, the oxidation state of Na⁺ is +1, and the oxidation state of Cl⁻ is -1.
-
The sum of the oxidation states of all atoms in a neutral molecule is 0.
-
The sum of the oxidation states of all atoms in a polyatomic ion is equal to the charge of the ion.
-
Hydrogen usually has an oxidation state of +1, except in metal hydrides (e.g., NaH), where it is -1.
-
Oxygen usually has an oxidation state of -2, except in peroxides (e.g., H₂O₂), where it is -1, and in superoxides (e.g., KO₂), where it is -1/2.
-
Fluorine always has an oxidation state of -1.
-
Other halogens (Cl, Br, I) usually have an oxidation state of -1, but can have positive oxidation states in compounds with more electronegative elements (e.g., oxygen).
-
Group 1 elements (alkali metals) always have an oxidation state of +1.
-
Group 2 elements (alkaline earth metals) always have an oxidation state of +2.
Analyzing Oxidation State Changes
Once you've assigned oxidation states to all atoms in both the reactants and products, compare the values. If you observe a change in the oxidation state of at least one atom, it's a redox reaction. A change indicates electron transfer.
Example 1: A Redox Reaction
Consider the reaction between zinc and copper(II) sulfate:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
- Reactants: Zn (0), Cu (+2), S (+6), O (-2)
- Products: Zn (+2), Cu (0), S (+6), O (-2)
Notice that the oxidation state of zinc increases from 0 to +2 (oxidation), while the oxidation state of copper decreases from +2 to 0 (reduction). This change in oxidation states confirms it's a redox reaction.
Example 2: Not a Redox Reaction
Consider the neutralization reaction between hydrochloric acid and sodium hydroxide:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
- Reactants: H (+1), Cl (-1), Na (+1), O (-2), H (+1)
- Products: Na (+1), Cl (-1), H (+1), O (-2)
In this reaction, there are no changes in the oxidation states of any atoms. Therefore, it is not a redox reaction. It's an acid-base reaction.
Method 2: Identifying Redox Reactions Using Half-Reactions
This method involves splitting the overall reaction into two half-reactions, one representing oxidation and the other representing reduction. If you can successfully separate the reaction into two half-reactions involving electron transfer, it's a redox reaction.
Balancing Half-Reactions
Balancing half-reactions requires careful attention to both mass and charge. You'll need to add electrons (e⁻) to balance the charge and adjust coefficients to balance the number of atoms of each element.
Example 3: A Redox Reaction (Half-Reaction Method)
Consider the reaction between iron(II) ions and permanganate ions in acidic solution:
MnO₄⁻(aq) + Fe²⁺(aq) → Mn²⁺(aq) + Fe³⁺(aq) (unbalanced)
This reaction can be separated into two half-reactions:
- Oxidation half-reaction: Fe²⁺(aq) → Fe³⁺(aq) + e⁻
- Reduction half-reaction: MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ → Mn²⁺(aq) + 4H₂O(l)
The presence of electrons in both half-reactions explicitly demonstrates electron transfer, confirming it's a redox reaction. The overall balanced equation is obtained by multiplying the oxidation half-reaction by 5 and adding it to the reduction half-reaction.
Method 3: Recognizing Characteristic Redox Reactions
While the oxidation state and half-reaction methods are rigorous, recognizing certain reaction patterns can help quickly identify redox reactions. These include:
-
Combustion reactions: Reactions involving rapid oxidation of a substance, usually with oxygen, producing heat and light. Examples include the burning of fuels (e.g., methane, propane).
-
Corrosion: The oxidation of metals, often involving oxygen and water, leading to the deterioration of the metal. Rusting of iron is a classic example.
-
Single displacement reactions: Reactions where a more reactive element displaces a less reactive element from a compound. For example, zinc reacting with copper(II) sulfate (Example 1).
-
Reactions involving strong oxidizing agents: Substances like potassium permanganate (KMnO₄), potassium dichromate (K₂Cr₂O₇), and hydrogen peroxide (H₂O₂) readily accept electrons and are frequently involved in redox reactions.
-
Reactions involving strong reducing agents: Substances like lithium (Li), sodium (Na), and magnesium (Mg) readily donate electrons and are frequently involved in redox reactions.
Common Pitfalls and Considerations
-
Beware of apparent redox reactions: Some reactions might appear to involve oxidation state changes but are not true redox reactions. These often involve covalent compounds where the oxidation state assignments are somewhat arbitrary. A thorough analysis is required.
-
Context matters: The identification of a redox reaction can depend on the specific reaction conditions (e.g., acidic, basic, neutral). The half-reactions and overall balanced equation might vary.
-
Complex reactions: In complex reactions with multiple reactants and products, carefully track the oxidation state changes of each atom to avoid errors.
Conclusion: Mastering Redox Reaction Identification
Identifying redox reactions is a cornerstone skill in chemistry. By mastering the methods outlined above – analyzing oxidation state changes, employing half-reactions, and recognizing characteristic reaction patterns – you’ll gain confidence in determining whether a chemical reaction involves the fundamental process of electron transfer. Remember that practice is key. The more redox reactions you analyze, the more intuitive the process will become. Always double-check your work, paying close attention to details, especially when dealing with complex chemical reactions. This comprehensive guide provides you with the tools you need to confidently navigate the fascinating world of redox chemistry.
Latest Posts
Latest Posts
-
How To Do Statistical Data Transformations In Excel
Mar 29, 2025
-
Why Are Hydrogen Bonds Important For Life
Mar 29, 2025
-
Amount Of Lime To Neutralie 9 Lbs Of Solfuric Acid
Mar 29, 2025
-
Why Are Base Pairing Rules Important
Mar 29, 2025
-
Use The Cofactor Expansion To Compute The Following Determinant
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Do You Know If A Reaction Is Redox . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
