How Does An Allosteric Inhibitor Work
Muz Play
Mar 20, 2025 · 6 min read
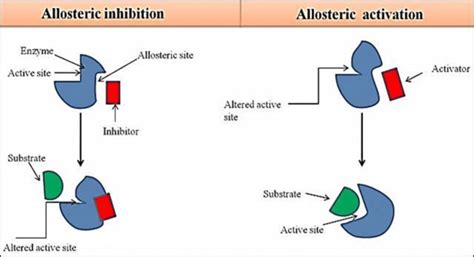
Table of Contents
How Does an Allosteric Inhibitor Work? A Deep Dive into Enzyme Regulation
Allosteric inhibitors are fascinating molecules that play a crucial role in regulating enzyme activity within living organisms. Understanding how they work is fundamental to comprehending cellular processes, developing pharmaceuticals, and advancing our knowledge of biochemistry. This in-depth article will explore the mechanisms of allosteric inhibition, its significance, and various examples.
Understanding Enzymes and Their Regulation
Before diving into allosteric inhibitors, let's briefly review the basics of enzymes. Enzymes are biological catalysts, proteins that significantly speed up the rate of chemical reactions within cells without being consumed in the process. They achieve this by binding to specific molecules called substrates, forming an enzyme-substrate complex that facilitates the reaction. This interaction typically occurs at a specific site on the enzyme known as the active site.
Enzyme activity isn't constant; it's finely regulated to meet the cell's changing needs. This regulation is essential for maintaining homeostasis and preventing unwanted reactions. Several mechanisms control enzyme activity, including:
- Allosteric regulation: This involves the binding of molecules to sites other than the active site, influencing the enzyme's shape and activity.
- Competitive inhibition: A molecule structurally similar to the substrate competes for binding to the active site.
- Non-competitive inhibition: A molecule binds to a site other than the active site, altering the enzyme's shape and reducing its activity. (Note: While this is also non-active site binding, the mechanism differs from allosteric inhibition in several key ways which will be discussed later).
- Feedback inhibition: The end product of a metabolic pathway inhibits an earlier enzyme in the pathway.
- Covalent modification: Chemical modifications, like phosphorylation, alter enzyme activity.
What are Allosteric Inhibitors?
Allosteric inhibitors are molecules that bind to a site on an enzyme distinct from the active site, known as the allosteric site. This binding causes a conformational change in the enzyme's three-dimensional structure, altering the shape of the active site and reducing or completely abolishing its ability to bind to the substrate. This ultimately decreases the enzyme's catalytic activity. Unlike competitive inhibitors, allosteric inhibitors don't directly compete with the substrate for the active site.
The Allosteric Site: A Key Player in Regulation
The allosteric site is a unique region on the enzyme possessing a specific binding pocket for the allosteric inhibitor. The binding of the inhibitor is often non-covalent, meaning it's reversible. The interaction strength is determined by several factors, including the inhibitor's structure, the enzyme's conformation, and the surrounding environment (e.g., pH, temperature).
Conformational Changes: The Mechanism of Inhibition
The binding of an allosteric inhibitor initiates a cascade of conformational changes within the enzyme. These changes can be subtle or dramatic, but they fundamentally alter the active site's structure. This alteration can manifest in several ways:
- Direct blockage: The conformational change might physically obstruct the substrate from entering the active site.
- Reduced affinity: The change could reduce the active site's affinity for the substrate, hindering the formation of the enzyme-substrate complex.
- Altered catalytic activity: Even if the substrate binds, the altered active site might be unable to catalyze the reaction efficiently.
Key Differences Between Allosteric and Competitive Inhibition
While both allosteric and competitive inhibitors reduce enzyme activity, they differ significantly in their mechanisms:
| Feature | Allosteric Inhibition | Competitive Inhibition |
|---|---|---|
| Binding Site | Allosteric site (separate from active site) | Active site |
| Mechanism | Conformational change affecting active site | Direct competition for active site |
| Effect on Vmax | Decreases Vmax (maximum reaction rate) | Decreases Vmax |
| Effect on Km | Can increase or decrease Km (substrate affinity) | Increases Km |
| Reversibility | Usually reversible | Usually reversible |
The differences in the effects on Vmax and Km are crucial for distinguishing between allosteric and competitive inhibition experimentally.
Types of Allosteric Inhibitors
Allosteric inhibitors can be broadly classified based on their effects on the enzyme:
-
Orthosteric Inhibitors: These inhibitors bind to the same site as the substrate (the active site) but do not directly compete with it. Instead, they alter the enzyme's conformation, making it less effective. While technically binding at the active site, their mechanism is more closely related to allosteric effects.
-
Non-Competitive Inhibitors: These inhibitors bind to an allosteric site and reduce the enzyme's activity without affecting substrate binding. They alter the Vmax but not the Km.
-
Mixed Inhibitors: These inhibitors bind to an allosteric site and both reduce the enzyme's activity and affect its substrate affinity (Km). They alter both the Vmax and Km.
Significance of Allosteric Inhibition in Biology and Medicine
Allosteric inhibition plays a vital role in regulating numerous cellular processes. Its significance is highlighted in several contexts:
Metabolic Regulation
Allosteric inhibition is crucial for regulating metabolic pathways. Feedback inhibition, a common example, prevents the overproduction of metabolic intermediates. The end product of a pathway often acts as an allosteric inhibitor of an earlier enzyme, ensuring a balanced supply of the final product.
Drug Design and Development
Allosteric inhibitors are increasingly important targets for drug development. They offer several advantages over competitive inhibitors:
- Higher specificity: Allosteric inhibitors often exhibit greater selectivity for their target enzymes, minimizing off-target effects.
- Potential for novel mechanisms of action: They can exploit unique conformational changes in enzymes to inhibit activity.
- Reduced resistance: Mutations that confer resistance to allosteric inhibitors may be less likely to occur compared to mutations conferring resistance to competitive inhibitors.
Examples in Medicine
Numerous drugs currently used in medicine are allosteric inhibitors, including:
- Antibiotics: Certain antibiotics target bacterial enzymes, inhibiting their activity and preventing bacterial growth.
- Cancer therapies: Some cancer drugs inhibit enzymes involved in cancer cell growth and proliferation.
- Treatment for neurological disorders: Allosteric inhibitors have shown promise in treating neurological disorders by modulating neurotransmitter activity.
Studying Allosteric Inhibition
Studying allosteric inhibitors requires a combination of biochemical and biophysical techniques:
- Enzyme kinetics: Measuring the reaction rate at varying substrate and inhibitor concentrations helps determine the type and strength of inhibition.
- X-ray crystallography: Determining the three-dimensional structure of the enzyme with and without the inhibitor reveals the conformational changes involved in inhibition.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR can provide detailed information about the dynamic interactions between the inhibitor and the enzyme.
- Computational modeling: Computer simulations can predict the binding affinities of potential inhibitors and the conformational changes they induce.
Future Directions in Allosteric Inhibition Research
The field of allosteric inhibition research continues to evolve rapidly. Future directions include:
- Developing more potent and selective allosteric inhibitors: This requires a deeper understanding of enzyme structure and dynamics.
- Exploring novel allosteric sites: Identifying new allosteric sites on enzymes could lead to the development of novel therapeutic agents.
- Utilizing advanced computational methods: Computational tools can play a significant role in designing and optimizing allosteric inhibitors.
- Investigating allosteric modulation in complex biological systems: Understanding allosteric regulation within complex networks is crucial for developing targeted therapies.
Conclusion
Allosteric inhibitors are remarkable molecules that exert precise control over enzyme activity, impacting diverse biological processes. Their complex mechanisms, specific binding characteristics, and diverse applications highlight their importance in biology, medicine, and various other scientific fields. The ongoing research in this area continues to unravel the intricate details of allosteric regulation, promising significant advancements in drug design and our understanding of life itself. The future of allosteric inhibition research holds immense potential for developing novel therapeutic strategies and further elucidating the intricate workings of enzymes within living systems.
Latest Posts
Latest Posts
-
Senate Races Tend To Inspire
Mar 21, 2025
-
The Mean Of The Sample Means
Mar 21, 2025
-
Is Sulfur More Electronegative Than Oxygen
Mar 21, 2025
-
Color The Neuron And Neuroglial Cells Answer Key
Mar 21, 2025
-
The Basic Unit Of Life Is The
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Does An Allosteric Inhibitor Work . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
