How Does Reactivity Of Metals Increase
Muz Play
Mar 30, 2025 · 5 min read
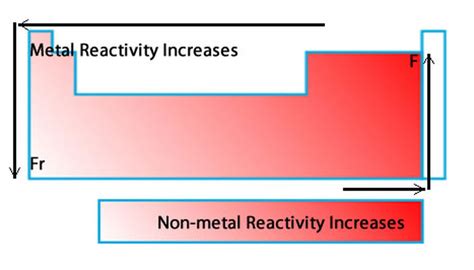
Table of Contents
How Does the Reactivity of Metals Increase? Understanding the Periodic Trends
The reactivity of metals is a fundamental concept in chemistry, influencing a vast range of applications from everyday life to advanced technologies. Understanding what drives this reactivity is crucial for predicting chemical behavior and designing new materials. This article delves deep into the factors that govern the increasing reactivity of metals, exploring the periodic trends, electron configurations, and the underlying principles that dictate their chemical behavior.
The Periodic Table: A Roadmap to Reactivity
The periodic table is our primary tool for understanding metallic reactivity. As we move down a group (vertical column) or across a period (horizontal row), the properties of elements, including their reactivity, change systematically.
Group Trends: The Downward Ascent of Reactivity
Within a group, reactivity generally increases as we go down. This is primarily due to the increasing distance of the valence electrons (outermost electrons) from the nucleus. Consider the alkali metals (Group 1):
- Lithium (Li): Its single valence electron is relatively close to the positively charged nucleus, experiencing a strong attraction. This makes it relatively less reactive.
- Sodium (Na): The valence electron in sodium is further from the nucleus, shielded by inner electron shells. This weaker attraction allows the electron to be more easily lost, leading to increased reactivity.
- Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr): Following the same trend, the reactivity continues to increase down the group as the valence electron becomes increasingly shielded and farther from the nucleus.
This effect is also observed in other metal groups, although the magnitude of the increase in reactivity might vary depending on the specific group and the electronic configurations involved.
Period Trends: The Rightward Decrease in Reactivity
Moving across a period from left to right, the reactivity of metals generally decreases. This is because the increasing nuclear charge attracts the valence electrons more strongly. As we add protons to the nucleus without significantly increasing the shielding effect, the electrons are held more tightly. This makes it harder for them to be lost, thus decreasing the metal's reactivity.
For example, comparing sodium (Na) to magnesium (Mg) and aluminum (Al) within the third period, we see a gradual decrease in reactivity. Sodium, with one valence electron, readily loses it to achieve a stable electron configuration. Magnesium, with two valence electrons, requires more energy to lose both electrons. Aluminum, with three valence electrons, exhibits even lower reactivity due to the increasing difficulty in losing three electrons.
The Role of Atomic Structure and Ionization Energy
The reactivity of metals is intrinsically linked to their atomic structure, specifically their electron configuration and ionization energy.
Electron Configuration: The Key to Reactivity
Metals are characterized by their tendency to lose electrons to achieve a stable electron configuration, typically resembling a noble gas (Group 18). The number of valence electrons determines how readily a metal loses electrons. Metals with fewer valence electrons generally exhibit higher reactivity because they require less energy to lose these electrons and attain a stable octet (or duet for hydrogen and helium).
Ionization Energy: The Energy Barrier to Reactivity
Ionization energy is the energy required to remove an electron from a gaseous atom. Lower ionization energy indicates higher reactivity because it means less energy is needed to remove an electron, making the metal more prone to lose electrons and participate in chemical reactions. Across a period, ionization energy increases, mirroring the decrease in reactivity. Down a group, ionization energy decreases, corresponding to the increased reactivity.
Factors Influencing Reactivity Beyond Periodic Trends
While periodic trends offer a general guideline, other factors can subtly influence the reactivity of metals:
Atomic Radius: Size Matters
Larger atomic radius generally correlates with higher reactivity. As the distance between the valence electrons and the nucleus increases, the attraction weakens, making it easier to lose electrons. This is consistent with the trend observed down a group.
Shielding Effect: The Inner Electrons' Role
Inner electrons shield the valence electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge experienced by the valence electrons, leading to weaker attraction and higher reactivity. This effect is crucial in explaining the increase in reactivity down a group.
Electronegativity: The Attraction to Electrons
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Metals have low electronegativity, meaning they are less likely to attract electrons and more likely to lose them, hence their reactive nature.
Practical Applications of Understanding Metal Reactivity
The knowledge of metal reactivity has profound implications across various fields:
- Corrosion: Understanding reactivity helps predict the corrosion susceptibility of metals. Highly reactive metals corrode more easily.
- Extraction of Metals: The reactivity of metals dictates the methods employed in their extraction from ores. Highly reactive metals require more energy-intensive extraction processes.
- Battery Technology: The reactivity of metals plays a crucial role in the design and performance of batteries. The choice of metals for electrodes is based on their redox potentials, which are directly related to their reactivity.
- Catalysis: Certain metals exhibit high catalytic activity due to their ability to readily lose and gain electrons. This is exploited in numerous industrial processes.
- Material Science: Understanding reactivity helps in designing alloys with specific properties, tailored for various applications.
Conclusion: A Dynamic Interplay of Forces
The reactivity of metals is not a static property but a dynamic interplay of various factors – electron configuration, ionization energy, atomic radius, shielding effect, and electronegativity. The periodic trends provide a valuable framework for understanding these interactions, enabling us to predict and manipulate the chemical behavior of metals for a wide array of applications. This fundamental knowledge continues to drive innovation in diverse fields, shaping our technological landscape and paving the way for future advancements. Further research into the nuances of metal reactivity promises to unlock even greater possibilities in materials science, energy technology, and beyond. Understanding how reactivity increases provides a foundational understanding of chemical behavior and its crucial role in our world.
Latest Posts
Latest Posts
-
Journal Entry To Issue Common Stock
Apr 01, 2025
-
Real Life Examples Of Linear Equations In Two Variable
Apr 01, 2025
-
What Does An Mean In Arithmetic Sequences
Apr 01, 2025
-
How To Find Pmf From Cdf
Apr 01, 2025
-
Area By Integration Problems With Solutions
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Does Reactivity Of Metals Increase . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
