How Many Covalent Bonds Does Hydrogen Have
Muz Play
Mar 20, 2025 · 5 min read
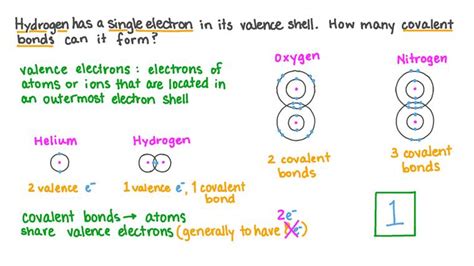
Table of Contents
How Many Covalent Bonds Does Hydrogen Have? A Deep Dive into Hydrogen Bonding
Hydrogen, the simplest and most abundant element in the universe, plays a crucial role in countless chemical reactions and biological processes. Understanding its bonding behavior is fundamental to grasping chemistry at a deeper level. This article delves into the intricacies of hydrogen bonding, specifically addressing the question: how many covalent bonds does hydrogen typically form?
The Uniqueness of Hydrogen
Hydrogen's unique position in the periodic table, with only one proton and one electron, dictates its bonding capabilities. Unlike many other elements capable of forming multiple bonds, hydrogen's capacity is limited by its single electron. This single electron is the key to understanding hydrogen's bonding behavior.
Electronic Configuration and Bonding
Hydrogen's electron configuration (1s<sup>1</sup>) means it has one electron in its outermost shell (valence shell). To achieve a stable electron configuration, resembling that of helium (1s<sup>2</sup>), hydrogen needs to either gain or lose an electron, or share an electron with another atom. This drive for stability is the fundamental driving force behind chemical bonding.
Hydrogen's Covalent Bonding Preference
Hydrogen predominantly forms covalent bonds. A covalent bond is formed when two atoms share a pair of electrons, allowing both atoms to effectively fill their valence shells. Because hydrogen has only one electron, it can only share one pair of electrons, meaning it typically forms one covalent bond.
Exceptions and Nuances: Beyond the Single Bond
While hydrogen typically forms only one covalent bond, there are some subtle exceptions and nuances to consider:
Hydrogen Bonds (A Special Case)
The term "hydrogen bond" is often confused with a covalent bond involving hydrogen. It's crucial to understand the difference. A hydrogen bond is a special type of dipole-dipole attraction between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. It is not a covalent bond. Hydrogen bonds are significantly weaker than covalent bonds. While not a covalent bond itself, the hydrogen bond's strength and directionality can profoundly influence the structure and properties of molecules, particularly in biological systems like DNA and proteins.
Dihydrogen Complexes: A Rare Occurrence
In certain specialized organometallic complexes, hydrogen can participate in what are known as dihydrogen complexes. In these complexes, a dihydrogen molecule (H<sub>2</sub>) acts as a ligand, coordinating to a metal center. The bond between the two hydrogen atoms is weakened, but it's important to note that this is not the formation of two separate covalent bonds by the hydrogen atoms. Instead, the dihydrogen molecule as a whole acts as a ligand, interacting with the metal. These are relatively rare and highly specialized cases.
Three-Center Two-Electron Bonds: Boron Compounds
In some boron compounds, hydrogen can participate in a three-center two-electron bond. This type of bond involves three atoms sharing two electrons. While unusual, this does not mean hydrogen forms two covalent bonds, but rather participates in a unique electron-sharing arrangement involving a delocalized bond. This is again a specialized situation, primarily encountered in the context of boron hydrides.
Illustrative Examples: Hydrogen's Covalent Bonding in Action
Let's look at some typical examples of hydrogen's covalent bonding:
Water (H₂O)
Each hydrogen atom in a water molecule forms one single covalent bond with the oxygen atom. The oxygen atom shares two electrons, one with each hydrogen, fulfilling the octet rule for oxygen and the duet rule for hydrogen.
Methane (CH₄)
In methane, each hydrogen atom forms one single covalent bond with the central carbon atom. The carbon atom shares one electron with each of the four hydrogen atoms.
Ammonia (NH₃)
Similar to methane and water, each hydrogen atom in ammonia forms a single covalent bond with the central nitrogen atom.
Hydrochloric Acid (HCl)
Hydrogen forms one covalent bond with chlorine in hydrochloric acid.
Why the Single Covalent Bond Predominates
The predominance of a single covalent bond for hydrogen stems directly from its electronic structure. With only one electron available for sharing, it's fundamentally limited to forming one covalent bond to achieve a stable electronic configuration. Any attempt to form more bonds would require exceeding its valence shell capacity.
Applications and Implications
Understanding hydrogen's bonding capacity is crucial for numerous applications across diverse scientific fields:
- Chemistry: Predicting reactivity, stability, and properties of molecules.
- Materials Science: Designing new materials with specific properties.
- Biochemistry: Understanding the structure and function of biological molecules.
- Energy: Developing hydrogen-based fuels and energy storage solutions.
Conclusion: One Bond, Many Implications
In summary, hydrogen typically forms only one covalent bond. While there are exceptions such as dihydrogen complexes and three-center two-electron bonds, these are highly specialized cases. The prevalence of a single covalent bond for hydrogen is a direct consequence of its electronic configuration and its drive to achieve a stable electronic structure. This seemingly simple bonding behavior has profound implications across numerous scientific disciplines and underscores the fundamental importance of hydrogen in chemistry and beyond. Understanding hydrogen's bonding capacity is vital for ongoing advancements in fields ranging from material science to biochemistry and beyond. The single covalent bond formed by hydrogen is a testament to the power of simplicity in the natural world, shaping the properties of countless molecules and driving the processes that sustain life itself.
Latest Posts
Latest Posts
-
What Is The Basic Unit Of Heredity
Mar 20, 2025
-
Why Is Equatorial More Stable Than Axial
Mar 20, 2025
-
Chemistry A Molecular Approach Nivaldo Tro
Mar 20, 2025
-
Humidity Is Measured With What Instrument
Mar 20, 2025
-
The Elite Theory Of Government Maintains That
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Covalent Bonds Does Hydrogen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
