How Many Electrons Does Na Have
Muz Play
Mar 22, 2025 · 5 min read
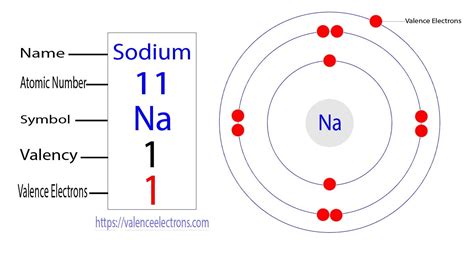
Table of Contents
How Many Electrons Does Na Have? Unveiling the Secrets of Sodium
Sodium (Na), a ubiquitous element found in table salt and essential for life, holds a fascinating place in the periodic table. Understanding its electron configuration is crucial to grasping its chemical behavior and reactivity. But the simple question, "How many electrons does Na have?" opens the door to a deeper exploration of atomic structure, electron shells, and the periodic trends that govern the properties of elements. This article will delve into the answer, exploring the fundamental concepts of atomic structure and providing a detailed explanation suitable for both beginners and those seeking a more thorough understanding.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we determine the number of electrons in a sodium atom, let's establish a foundational understanding of atomic structure. An atom is the basic building block of matter, composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus (center). The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons are responsible for the chemical properties of an element. The number of electrons in a neutral atom is equal to the number of protons.
Sodium's Position in the Periodic Table: A Clue to its Electron Count
The periodic table organizes elements based on their atomic number and recurring chemical properties. Sodium (Na) is located in Group 1 (also known as Alkali Metals) and Period 3. Its atomic number is 11. This atomic number directly tells us the number of protons in a sodium atom: 11 protons.
Since a neutral atom has an equal number of protons and electrons, a neutral sodium atom possesses 11 electrons.
Electron Shells and Subshells: A Detailed Look at Sodium's Electron Configuration
Electrons don't orbit the nucleus randomly. They occupy specific energy levels, often visualized as shells or orbitals. These shells are designated by principal quantum numbers (n = 1, 2, 3, etc.), with each shell capable of holding a maximum number of electrons.
- Shell 1 (n=1): Can hold a maximum of 2 electrons.
- Shell 2 (n=2): Can hold a maximum of 8 electrons.
- Shell 3 (n=3): Can hold a maximum of 18 electrons.
Sodium's 11 electrons are distributed among these shells as follows:
- Shell 1: 2 electrons
- Shell 2: 8 electrons
- Shell 3: 1 electron
This distribution is commonly represented as the electron configuration: 1s²2s²2p⁶3s¹.
- 1s²: Two electrons in the 1s subshell (lowest energy level).
- 2s²: Two electrons in the 2s subshell.
- 2p⁶: Six electrons in the 2p subshell (three orbitals, each holding two electrons).
- 3s¹: One electron in the 3s subshell (outermost shell).
This outermost electron in the 3s subshell is what determines sodium's reactivity and its placement in Group 1 (Alkali Metals). Alkali metals are highly reactive due to their tendency to lose this single valence electron to achieve a stable electron configuration, resembling the noble gas neon (Ne).
Isotopes of Sodium: Variations in Neutron Count, but Consistent Electron Number
While the number of electrons in a neutral sodium atom remains consistently 11, the number of neutrons can vary, leading to isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotope of sodium is Sodium-23 (²³Na), with 11 protons and 12 neutrons. However, other less abundant isotopes of sodium exist, such as Sodium-22 (²²Na). Regardless of the isotope, the number of electrons in a neutral sodium atom always remains 11.
The Significance of Sodium's Electron Configuration in its Chemical Behavior
Sodium's electron configuration explains its remarkable reactivity:
- Ease of Ionization: The single electron in the outermost shell (3s¹) is relatively loosely held. It requires relatively little energy to remove this electron, forming a positively charged sodium ion (Na⁺). This ease of ionization contributes to sodium's high reactivity.
- Formation of Ionic Compounds: Sodium readily loses its valence electron to achieve a stable octet (eight electrons) in its outermost shell, resembling the noble gas neon. This electron transfer forms ionic bonds with other elements, particularly nonmetals such as chlorine (Cl), resulting in the formation of ionic compounds like sodium chloride (NaCl), commonly known as table salt.
- Reduction Reactions: Because sodium readily loses an electron, it acts as a reducing agent in chemical reactions, donating electrons to other substances. This property is crucial in various chemical processes and industrial applications.
Sodium's Role in Biology and Everyday Life
Understanding sodium's electron configuration allows us to appreciate its vital role in biological systems and everyday life:
- Electrolyte Balance: Sodium ions (Na⁺) are essential electrolytes in maintaining fluid balance, nerve impulse transmission, and muscle contraction in living organisms.
- Table Salt: Sodium chloride (NaCl), a common compound in our diet, plays a crucial role in flavoring food. However, excessive sodium intake can be detrimental to health.
- Industrial Applications: Sodium is used in various industrial processes, including the production of other chemicals, soaps, and detergents. Its reactivity and ability to conduct electricity make it valuable in different industries.
Conclusion: Understanding the Significance of 11 Electrons
The seemingly simple question, "How many electrons does Na have?" has led us on a journey through the fascinating world of atomic structure, electron configurations, and chemical reactivity. The answer, 11 electrons, is more than just a number; it's the key to understanding sodium's unique properties, its chemical behavior, and its crucial role in the natural world. This understanding underscores the fundamental importance of atomic structure in determining the properties and applications of elements, a principle crucial to many fields of science and technology. The 11 electrons in sodium aren't just particles; they represent the essence of this vital element and its influence on our lives.
Latest Posts
Latest Posts
-
A Solution Of Salt In Water Is A
May 09, 2025
-
Explain Why Organisms Need Enzymes To Survive
May 09, 2025
-
Choose The Correct Name For The Following Amine
May 09, 2025
-
What Is The Unifying Principle Of The Biological Sciences
May 09, 2025
-
The Study Of Matter And The Changes It Undergoes
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Na Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.