How Many Valence Electrons Does Sulfer Have
Muz Play
Apr 05, 2025 · 6 min read
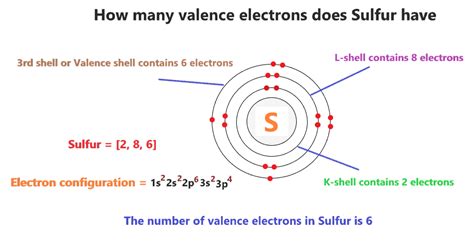
Table of Contents
- How Many Valence Electrons Does Sulfer Have
- Table of Contents
- How Many Valence Electrons Does Sulfur Have? A Deep Dive into Sulfur's Electronic Structure
- Understanding Valence Electrons
- Determining Sulfur's Valence Electrons
- Electronic Configuration and Valence Shell
- The Significance of Six Valence Electrons
- Sulfur's Chemical Reactivity
- Sulfur's Diverse Applications: A Consequence of its Electronic Structure
- Industrial Applications:
- Biological Roles:
- Beyond the Basics: Exploring Sulfur's Allotropes
- Conclusion: The Central Role of Six Valence Electrons
- Latest Posts
- Latest Posts
- Related Post
How Many Valence Electrons Does Sulfur Have? A Deep Dive into Sulfur's Electronic Structure
Sulfur, a vibrant yellow nonmetal found abundantly in nature, plays a crucial role in various biological and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is key to grasping its reactivity and diverse chemical behavior. This comprehensive guide delves into the intricacies of sulfur's electronic configuration, explaining why it possesses the number of valence electrons it does and how this impacts its chemical properties.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in sulfur, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of compounds it can form. They're the driving force behind chemical interactions, dictating how atoms interact to form molecules and compounds. The number of valence electrons an atom possesses directly correlates to its position on the periodic table and its group number.
Determining Sulfur's Valence Electrons
Sulfur (S) is located in Group 16 (also known as Group VIA) of the periodic table. The group number for the main group elements (those not in the transition metal blocks) generally indicates the number of valence electrons. Therefore, sulfur, being in Group 16, possesses six valence electrons.
Electronic Configuration and Valence Shell
To understand this more deeply, let's examine sulfur's electronic configuration. The electronic configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Sulfur's atomic number is 16, meaning it has 16 electrons. Its electronic configuration is 1s²2s²2p⁶3s²3p⁴.
- 1s², 2s², 2p⁶: These inner shell electrons are tightly bound to the nucleus and do not participate significantly in chemical bonding. They are considered core electrons.
- 3s², 3p⁴: These electrons reside in the outermost shell (the third energy level), making them the valence electrons. The 3s sublevel contains two electrons, and the 3p sublevel contains four electrons. Therefore, the total number of valence electrons is 2 + 4 = 6.
The Significance of Six Valence Electrons
The fact that sulfur has six valence electrons significantly influences its chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (elements in Group 18 with full outer electron shells). Sulfur can achieve stability by either gaining two electrons to complete its outermost shell (achieving an octet, like Argon) or by sharing electrons in covalent bonds.
Sulfur's Chemical Reactivity
This drive for stability explains sulfur's reactivity:
-
Formation of Anions: Sulfur readily gains two electrons to form the sulfide anion (S²⁻). This process is known as reduction, and the resulting sulfide ion has a stable octet of electrons. This explains the formation of ionic compounds such as sodium sulfide (Na₂S) where sulfur exists as a negatively charged ion.
-
Formation of Covalent Bonds: Alternatively, sulfur can share its six valence electrons to form covalent bonds with other atoms. This is particularly common with nonmetals. Sulfur can form single, double, or even multiple bonds, leading to a vast array of sulfur-containing molecules and compounds. Examples include hydrogen sulfide (H₂S), sulfur dioxide (SO₂), and sulfur trioxide (SO₃). The ability to form multiple bonds is a key characteristic of sulfur's chemistry, giving rise to the diverse range of molecules it is a part of.
Sulfur's Diverse Applications: A Consequence of its Electronic Structure
Sulfur's six valence electrons are at the heart of its diverse applications across various industries and biological systems. The ability to easily gain or share electrons leads to the formation of numerous compounds with unique properties.
Industrial Applications:
-
Sulfuric Acid Production: Sulfur is the primary raw material for sulfuric acid (H₂SO₄) production, one of the most important industrial chemicals globally. Its use spans various sectors, including fertilizers, detergents, and the production of other chemicals. The reactivity of sulfur, arising from its six valence electrons, is pivotal in these processes.
-
Rubber Vulcanization: Sulfur plays a crucial role in the vulcanization of rubber, a process that significantly improves the rubber's strength, elasticity, and durability. The cross-linking of rubber chains through sulfur bridges is a direct result of sulfur's ability to form covalent bonds.
-
Manufacturing of Matches and Fireworks: The combustion properties of sulfur make it a crucial component in matches and fireworks, contributing to their characteristic flames.
Biological Roles:
-
Amino Acids: Sulfur is a constituent element of two essential amino acids: cysteine and methionine. These amino acids are fundamental building blocks of proteins, playing crucial roles in protein structure and function. The sulfur atoms in these amino acids participate in disulfide bond formation, which is critical for the three-dimensional structure of many proteins.
-
Enzymes: Sulfur is present in several enzymes, where it participates in catalysis and other biochemical reactions. Its presence in these enzymes contributes to their catalytic activity and ability to accelerate biochemical reactions.
-
Antioxidants: Some sulfur-containing compounds have antioxidant properties, protecting cells from damage caused by free radicals. This antioxidant activity contributes to overall cellular health and protection against oxidative stress.
Beyond the Basics: Exploring Sulfur's Allotropes
Sulfur exhibits allotropy, meaning it can exist in different forms with varying physical properties. The most common allotrope is orthorhombic sulfur, but other forms exist, including monoclinic sulfur and various polymeric forms. These different allotropes arise from variations in how sulfur atoms bond to each other, all ultimately linked to the properties stemming from its six valence electrons. The arrangement of these electrons within the molecules of different sulfur allotropes gives rise to distinct structural and physical properties. This highlights how the basic electronic configuration can lead to a surprising variety of physical states for the same element.
Conclusion: The Central Role of Six Valence Electrons
The number of valence electrons an atom possesses is fundamental in determining its chemical behavior. Sulfur, with its six valence electrons, exemplifies this principle perfectly. Its ability to gain two electrons to form anions or to share electrons to form covalent bonds underpins its extensive reactivity and the vast array of compounds it forms. This reactivity explains sulfur's significant roles in various industrial applications and biological processes. Understanding sulfur's electronic structure, particularly its six valence electrons, is key to appreciating its significance in the natural world and its applications in diverse fields. From the production of sulfuric acid to the structure of proteins, the impact of sulfur's six valence electrons is undeniable. This comprehensive exploration showcases how a seemingly simple number can dictate the complex and fascinating chemistry of an element as vital as sulfur.
Latest Posts
Latest Posts
-
How To Draw Newman Projections From Chair Conformation
Apr 10, 2025
-
Electric Field Lines Vs Equipotential Lines
Apr 10, 2025
-
Which Of The Following Is An Oxidation Reaction
Apr 10, 2025
-
Elements That Are A Gas At Room Temperature
Apr 10, 2025
-
Process By Which A Cell Expels Wastes From A Vacuole
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Sulfer Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.