How To Determine A Strong Acid
Muz Play
Mar 29, 2025 · 6 min read
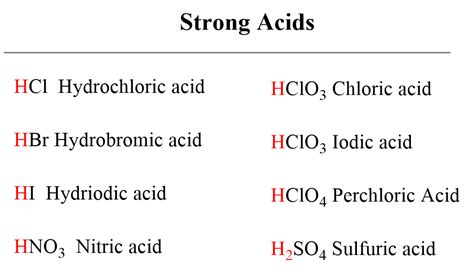
Table of Contents
How to Determine a Strong Acid: A Comprehensive Guide
Determining whether an acid is strong or weak is crucial in various fields, from chemistry and environmental science to medicine and engineering. Understanding the properties and behavior of strong acids is essential for accurate predictions and safe handling in laboratory settings and industrial processes. This comprehensive guide delves into the methods and concepts involved in identifying strong acids, ensuring a thorough understanding of this important topic.
Understanding the Definition of a Strong Acid
Before diving into the methods of identification, it's crucial to define what constitutes a strong acid. A strong acid is defined as an acid that completely dissociates (ionizes) in an aqueous solution. This means that when a strong acid is dissolved in water, virtually all of its molecules break apart into their constituent ions – hydrogen ions (H⁺) and an anion. This complete dissociation is a key characteristic that distinguishes strong acids from weak acids, which only partially dissociate.
The complete dissociation of a strong acid is represented by a single arrow (→) in a chemical equation, indicating an irreversible reaction. For example, the dissociation of hydrochloric acid (HCl), a common strong acid, is represented as:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Key Properties of Strong Acids
Strong acids exhibit several key properties that can be used to help identify them:
1. High Acidity (Low pH):
Strong acids have a very low pH, typically below 1. This is because of the high concentration of H⁺ ions in the solution. The pH scale is logarithmic, meaning a change of one pH unit represents a tenfold change in H⁺ ion concentration. Therefore, a pH of 1 indicates a significantly higher H⁺ concentration than a pH of 2, for instance. A pH meter is a useful instrument to measure the acidity.
2. Complete Dissociation:
As mentioned earlier, the defining characteristic of a strong acid is its complete dissociation into ions in water. This means that the concentration of the undissociated acid molecules is negligible compared to the concentration of the ions.
3. High Electrical Conductivity:
Strong acids are excellent conductors of electricity. This is because the high concentration of mobile ions (H⁺ and the anion) in the solution allows for easy passage of electrical current. This conductivity can be tested using a conductivity meter.
4. Reactivity with Metals:
Strong acids react vigorously with many metals, producing hydrogen gas (H₂) and a metal salt. The reactivity varies depending on the metal's position in the reactivity series. For example, the reaction between hydrochloric acid and zinc is a classic example:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g)
5. Reaction with Bases:
Strong acids readily react with bases in a neutralization reaction, forming water and a salt. The reaction is highly exothermic, often releasing significant heat. This is a common method used to quantify the concentration of an unknown acid through titration.
Methods for Determining if an Acid is Strong
Several methods can be employed to determine if an acid is strong. These methods range from simple observations to more sophisticated laboratory techniques:
1. pH Measurement:
The simplest method is to measure the pH of an aqueous solution of the acid using a pH meter or pH indicator paper. A very low pH (typically below 1) strongly suggests a strong acid. However, this method alone isn't conclusive because some weak acids can also have relatively low pH values if they are highly concentrated.
2. Conductivity Measurement:
Measuring the electrical conductivity of the solution provides another clue. Strong acids exhibit high conductivity due to the high concentration of ions. A conductivity meter can measure the conductivity quantitatively. Again, this isn't definitive proof, as highly concentrated solutions of some weak acids might also exhibit relatively high conductivity.
3. Titration:
Titration is a quantitative method to determine the concentration of an acid (or base) by reacting it with a solution of a base (or acid) of known concentration. By monitoring the pH change during the titration, one can determine the equivalence point, which provides information about the acid's concentration and strength. Strong acids show a sharp pH change near the equivalence point during titration, unlike weak acids.
4. Spectroscopic Analysis:
Sophisticated techniques like infrared (IR) spectroscopy or nuclear magnetic resonance (NMR) spectroscopy can be used to analyze the chemical structure and identify the presence of undissociated acid molecules. A strong acid will show minimal or no presence of undissociated molecules in an aqueous solution, confirming complete dissociation.
5. Thermodynamic Considerations:
The strength of an acid is fundamentally related to its equilibrium constant (Ka) for dissociation. A strong acid has a very large Ka value, implying that the equilibrium strongly favors the dissociated form. The pKa (negative logarithm of Ka) is often used to express this; strong acids have very low pKa values. While directly measuring Ka or pKa requires advanced techniques, literature values are readily available for many common acids.
Common Strong Acids
It's beneficial to familiarize yourself with the common strong acids frequently encountered in chemistry:
- Hydrochloric acid (HCl): Found in gastric acid and used in many industrial processes.
- Sulfuric acid (H₂SO₄): Used extensively in industrial applications, including fertilizer production and petroleum refining.
- Nitric acid (HNO₃): Used in the production of fertilizers and explosives.
- Hydrobromic acid (HBr): Less commonly used but still considered a strong acid.
- Hydroiodic acid (HI): Similar to HBr, it's a strong acid.
- Perchloric acid (HClO₄): A very strong acid, often used in analytical chemistry.
Important Safety Precautions
When handling strong acids, it is crucial to follow appropriate safety precautions:
- Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat.
- Work in a well-ventilated area to avoid inhalation of acid fumes.
- Never directly smell or taste an acid.
- Always add acid to water, not water to acid, to prevent splashing and potentially dangerous exothermic reactions.
- Neutralize spills immediately using a suitable base, such as sodium bicarbonate.
- Dispose of acid waste properly according to established safety protocols.
Conclusion
Determining whether an acid is strong involves understanding its defining properties – complete dissociation in water and consequently, high acidity, high conductivity, and reactivity. Various methods exist for this determination, from simple pH measurements and conductivity tests to more sophisticated techniques like titration and spectroscopic analysis. While a low pH is suggestive, a combination of tests provides the most reliable confirmation. Always remember to prioritize safety when working with acids, especially strong acids, and adhere strictly to appropriate safety guidelines. This comprehensive understanding is essential for anyone working with acids, whether in a laboratory, industrial setting, or educational environment. Careful observation, combined with appropriate analytical techniques, will ensure accurate identification and safe handling of these powerful chemical substances.
Latest Posts
Latest Posts
-
Solving Square Root And Other Radical Equations
Mar 31, 2025
-
What Are Characteristics Of A Virus
Mar 31, 2025
-
The Adrenal Glands Are Attached Superiorly To Which Organ
Mar 31, 2025
-
The Set Of Ordered Pairs That Defines The Relation
Mar 31, 2025
-
System Of Equations With 3 Variables
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Determine A Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
