How To Determine State Of Matter In A Chemical Equation
Muz Play
Mar 27, 2025 · 6 min read
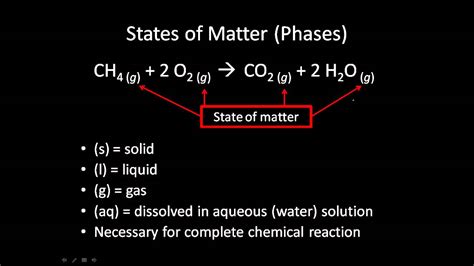
Table of Contents
How to Determine the State of Matter in a Chemical Equation
Determining the state of matter of reactants and products in a chemical equation is crucial for a complete and accurate representation of a chemical reaction. It provides valuable insight into the reaction's conditions, mechanism, and even its spontaneity. This article will explore various methods and considerations for correctly identifying and representing the physical state of substances within a chemical equation.
Understanding States of Matter
Before delving into the methods of determining the state of matter, it's essential to understand the common abbreviations used to represent these states:
- (s): Solid – A substance with a definite shape and volume. The particles are tightly packed and have strong intermolecular forces.
- (l): Liquid – A substance with a definite volume but an indefinite shape. Particles are closely packed but can move past each other.
- (g): Gas – A substance with an indefinite shape and volume. Particles are widely dispersed and have weak intermolecular forces. They are easily compressible.
- (aq): Aqueous – A substance dissolved in water. This is a special case, as the substance itself might be solid, liquid, or gas in its pure form, but its state within the reaction is dissolved in water.
Methods for Determining States of Matter
There are several ways to determine the state of matter of a substance in a chemical reaction:
1. Knowing the Properties of Substances:
This is the most fundamental approach. Familiarity with the physical and chemical properties of common substances is key. For example:
- At room temperature and pressure: You would expect substances like sodium chloride (NaCl) to be solid (s), water (H₂O) to be liquid (l), and oxygen (O₂) to be gas (g).
- Melting and Boiling Points: Knowing the melting and boiling points of a substance helps determine its state at a given temperature. If the temperature is below the melting point, it's a solid; between melting and boiling points, it's a liquid; and above the boiling point, it's a gas.
- Chemical Context: The context of the reaction can provide clues. For instance, a reaction occurring at high temperatures might involve substances in gaseous states even if they are typically solid or liquid at room temperature.
Example: Consider the reaction between hydrochloric acid and sodium hydroxide:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, we know that hydrochloric acid and sodium hydroxide are typically dissolved in water (aq), and the product, sodium chloride, is also soluble in water. However, water itself, even as a product, remains in its liquid state at normal conditions.
2. Using Experimental Observations:
Direct observation during the experiment is another valuable tool. You can visually determine the state of reactants and products. For instance:
- Precipitation reactions: The formation of a solid precipitate from a reaction between two aqueous solutions is clearly visible.
- Gas evolution: The release of a gas, indicated by bubbling or fizzing, identifies a gaseous product.
- Phase changes: Changes in state, such as melting or boiling, can be readily observed.
Example: The reaction between zinc and hydrochloric acid:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Observing the bubbling gas (H₂) confirms its gaseous state. The solid zinc (Zn) is visible as a reactant.
3. Referencing Standard Data Tables and Resources:
Comprehensive chemistry handbooks, textbooks, and online databases provide information on the physical properties of numerous substances, including their states at standard temperature and pressure (STP). These are extremely helpful for unfamiliar substances.
Important Note: Standard conditions are usually defined as 25°C (298 K) and 1 atm pressure. However, reaction conditions can significantly deviate from STP. Always consider the reaction's specific conditions when determining the state of matter.
4. Considering Reaction Conditions:
The temperature and pressure under which a reaction takes place strongly influence the state of matter of substances involved. High temperatures can vaporize solids or liquids, while low temperatures can solidify gases or liquids. High pressure can liquefy gases.
Example: The Haber-Bosch process for ammonia synthesis:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
This reaction occurs at high temperatures (around 450°C) and high pressures (around 200 atm) to favor the formation of ammonia gas. Under normal conditions, ammonia would exist as a gas, but the high pressure influences the equilibrium.
5. Understanding Reaction Mechanisms and Equilibrium:
The reaction mechanism and equilibrium constants can indirectly influence the determination of the state of matter. For instance, if a reaction involves the formation of an intermediate compound that is unstable at room temperature and quickly decomposes into gaseous products, then its initial state might not be directly observable, but its influence is implied in the final state of the products.
Example: The decomposition of hydrogen peroxide:
2H₂O₂(aq) → 2H₂O(l) + O₂(g)
While the initial state of hydrogen peroxide is aqueous, the mechanism involves the formation of unstable intermediates that lead to the release of oxygen gas.
Common Pitfalls and Considerations
- Ambiguity: Some substances can exist in multiple states depending on the conditions. Always specify the conditions (temperature, pressure) if there's any ambiguity.
- Ionic Compounds in Aqueous Solutions: Always indicate the (aq) state for ionic compounds dissolved in water.
- Complex Reactions: For complex reactions with multiple steps, determining the state of matter for each intermediate might require a deeper understanding of the reaction mechanism.
- Non-ideal Behavior: The ideal gas law provides an approximation. Under extreme conditions (high pressure, low temperature), real gases may deviate significantly from ideal behavior, making state determination more complex.
Practical Application: Worked Examples
Let's consider a few examples to solidify our understanding:
Example 1: Write the balanced chemical equation for the combustion of methane, specifying the states of matter.
The combustion of methane involves reacting methane (CH₄) gas with oxygen (O₂) gas to produce carbon dioxide (CO₂) gas and water (H₂O) vapor. Since the reaction is typically carried out at high temperatures, water exists as a gas.
Balanced Equation: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
Example 2: Write the balanced chemical equation for the reaction between aqueous solutions of lead(II) nitrate and potassium iodide, including states of matter.
This is a precipitation reaction where lead(II) iodide (PbI₂) precipitates out as a yellow solid.
Balanced Equation: Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq)
Example 3: The reaction between calcium carbonate (a solid) and hydrochloric acid (an aqueous solution) produces calcium chloride, carbon dioxide gas, and water. Write the balanced chemical equation.
Balanced Equation: CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
Conclusion
Accurately determining the state of matter in a chemical equation is essential for a complete and accurate representation of the chemical process. Combining knowledge of chemical properties, experimental observations, standard data tables, consideration of reaction conditions, and understanding reaction mechanisms are crucial for achieving this. By carefully applying these methods, we can gain a deeper understanding of chemical reactions and their outcomes. Remember to always consider the specific conditions of the reaction to avoid ambiguities and ensure the accuracy of your representation. Practicing with various examples will enhance your proficiency in this critical aspect of chemical notation.
Latest Posts
Latest Posts
-
What Directly Causes The Athenians To Hide In Their Homes
Mar 30, 2025
-
Differentiate Between Isometric And Isotonic Contractions
Mar 30, 2025
-
Match Each Equation With A Graph Above
Mar 30, 2025
-
How Do Organisms Generate Energy When Oxygen Is Not Available
Mar 30, 2025
-
What Does Lda Do In A Reaction
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Determine State Of Matter In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
