How To Find A Coordination Number
Muz Play
Apr 07, 2025 · 6 min read
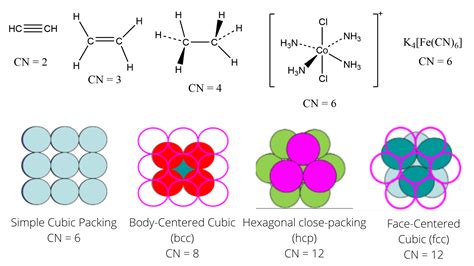
Table of Contents
How to Find a Coordination Number: A Comprehensive Guide
Determining the coordination number of a central atom in a molecule or crystal structure is a fundamental concept in chemistry and materials science. Understanding coordination numbers is crucial for predicting the properties and behaviors of various compounds. This comprehensive guide will delve into the intricacies of determining coordination numbers, covering various techniques and providing clear examples.
What is a Coordination Number?
The coordination number (CN) describes the number of atoms, molecules, or ions directly bonded to a central atom in a complex or crystal lattice. It's a key parameter in describing the geometry and properties of a compound. For instance, a coordination number of 4 might indicate a tetrahedral geometry, while a coordination number of 6 could suggest an octahedral geometry. However, this is a simplification and the actual geometry is influenced by many factors, including ligand size and electronic effects.
The coordination number isn't just relevant to discrete molecules; it plays a vital role in understanding crystalline solids. In crystals, the coordination number represents the number of nearest neighboring atoms surrounding a specific atom in the lattice structure. This influences the overall stability and properties of the solid.
Methods for Determining Coordination Number
Determining the coordination number requires careful consideration of the compound's structure. Different techniques can be employed depending on the availability of structural information.
1. From Molecular Geometry (Discrete Molecules):
For discrete molecules, determining the coordination number is relatively straightforward. It's simply a matter of counting the number of atoms directly bonded to the central atom. Let's illustrate this with some examples:
- Example 1: Methane (CH₄): The carbon atom is surrounded by four hydrogen atoms. Therefore, the coordination number of carbon in methane is 4.
- Example 2: Ammonia (NH₃): The nitrogen atom is bonded to three hydrogen atoms. Thus, the coordination number of nitrogen in ammonia is 3.
- Example 3: Water (H₂O): The oxygen atom is bonded to two hydrogen atoms. The coordination number of oxygen in water is 2.
- Example 4: Complex Ions: Consider the complex ion [Fe(CN)₆]³⁻. The central iron atom is bonded to six cyanide (CN⁻) ligands. The coordination number of iron in this complex is 6. Note that we count the number of ligands, not the number of individual atoms within the ligands.
2. From Crystal Structures (Solid State):
Determining coordination numbers in crystalline solids requires analyzing the crystal lattice structure. This often involves techniques such as X-ray diffraction, neutron diffraction, or electron diffraction. The analysis reveals the arrangement of atoms and the distances between them. The coordination number is determined by identifying the nearest neighboring atoms surrounding a particular atom within the lattice.
- Example 1: Simple Cubic Structure: In a simple cubic lattice, each atom is surrounded by 6 nearest neighbors. Therefore, the coordination number is 6.
- Example 2: Body-Centered Cubic (BCC) Structure: In a BCC structure, each atom has 8 nearest neighbors, resulting in a coordination number of 8.
- Example 3: Face-Centered Cubic (FCC) Structure: An FCC structure has a coordination number of 12, with each atom surrounded by 12 nearest neighbors.
These are examples of simple crystal structures. More complex structures will require more sophisticated analysis to determine the coordination number accurately. The coordination number can vary for different atoms within the same crystal structure, depending on their position within the lattice.
3. Using Software and Databases:
Modern computational tools and crystallographic databases can significantly simplify the process of determining coordination numbers. Software packages can analyze crystal structure data and automatically calculate coordination numbers for each atom in the structure. Databases like the Crystallography Open Database (COD) provide access to a vast collection of experimentally determined crystal structures, making it easier to find and analyze data for various compounds.
4. Considering Different Definitions:
It's important to acknowledge that different definitions of "nearest neighbor" can sometimes lead to slightly varying coordination numbers. For example, if the distances to neighboring atoms are very similar, defining the exact cutoff for "nearest neighbor" might be ambiguous. This usually isn't a major problem, but it's something to keep in mind when comparing coordination numbers from different sources.
Factors Influencing Coordination Number
Several factors influence the coordination number of an atom:
- Size of the central atom: Larger central atoms can accommodate more ligands or neighboring atoms, leading to higher coordination numbers.
- Size of the ligands: Larger ligands might sterically hinder the approach of additional ligands, resulting in lower coordination numbers.
- Charge of the central atom and ligands: Electrostatic interactions between the central atom and ligands play a crucial role. Higher charges generally lead to higher coordination numbers, provided that steric factors don't interfere.
- Electronic configuration: The electronic configuration of the central atom affects the number of bonds it can form, influencing the coordination number. Transition metal ions often exhibit variable coordination numbers due to their ability to form multiple bonds.
- Crystal packing: In solid-state structures, the efficient packing of atoms within the crystal lattice influences the coordination numbers.
Applications of Coordination Number
Understanding coordination numbers has significant applications across various scientific fields:
- Material Science: Predicting and controlling the properties of materials often requires understanding the coordination environments of atoms within the material's structure. Coordination number impacts mechanical strength, conductivity, and other physical properties.
- Catalysis: Coordination number plays a crucial role in catalytic activity. The coordination environment around the active site influences its reactivity and selectivity.
- Geochemistry: Coordination numbers are fundamental to understanding the structures and properties of minerals and other geological materials. They are crucial in analyzing the behavior of elements in various geological processes.
- Inorganic Chemistry: The coordination number is a cornerstone of understanding the structure and bonding in coordination complexes and inorganic compounds. It helps predict reactivity and stability.
- Biochemistry: Many biological molecules involve metal ions in specific coordination environments, which are vital for their function. Examples include enzymes and oxygen-carrying proteins.
Advanced Concepts and Considerations
While the basic principles of determining coordination numbers are relatively straightforward, several more complex scenarios exist:
- Variable Coordination Numbers: Some atoms can exhibit variable coordination numbers depending on the specific environment or bonding situation. This is particularly true for transition metals.
- Distorted Geometries: In some cases, the geometry around a central atom might be distorted from idealized geometries (e.g., tetrahedral, octahedral). This distortion can affect the interpretation of the coordination number.
- Coordination Polymers: Coordination polymers are extended structures where metal centers are linked by bridging ligands. Determining the coordination number in these complex structures can be challenging.
- Mixed Ligand Complexes: Complexes with multiple different types of ligands can have less symmetrical coordination environments, making the assignment of the coordination number more nuanced.
Conclusion
Determining the coordination number is a crucial aspect of understanding the structure and properties of molecules and materials. This guide has provided a comprehensive overview of different techniques and considerations involved in determining coordination numbers, encompassing both simple molecular structures and complex crystalline solids. By mastering these concepts, researchers and students can gain valuable insights into the intricacies of chemical bonding and material behavior. The ability to accurately determine coordination numbers is essential for advancing research in various fields, from materials science and catalysis to geochemistry and biochemistry. Remember to consider the factors influencing coordination numbers and utilize available tools and databases to facilitate the process and ensure accuracy.
Latest Posts
Latest Posts
-
Why Is Prophase The Longest Phase Of Mitosis
Apr 10, 2025
-
What Are Four Basic Economic Questions
Apr 10, 2025
-
Name The Ionic Compound Mgcl2
Apr 10, 2025
-
Activity Three Modes Of Natural Selection
Apr 10, 2025
-
Work Equilibrium And Free Energy Pogil Answer Key
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about How To Find A Coordination Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.