How To Find Protons Neutrons And Electrons Of Isotopes
Muz Play
Apr 07, 2025 · 5 min read
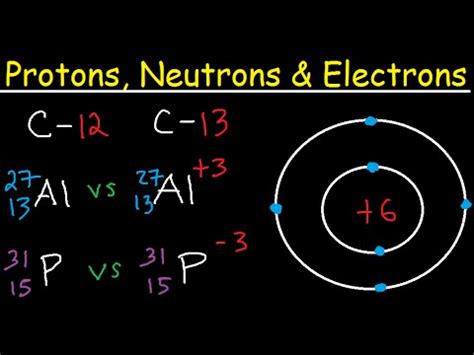
Table of Contents
How to Find Protons, Neutrons, and Electrons in Isotopes
Understanding the composition of atoms, particularly isotopes, is fundamental to chemistry and physics. This comprehensive guide will walk you through the process of determining the number of protons, neutrons, and electrons in various isotopes. We'll cover the basics, delve into the nuances of isotopes, and equip you with the skills to confidently tackle these calculations.
Understanding Atomic Structure: The Building Blocks
Before we explore isotopes, let's establish a firm grasp of basic atomic structure. An atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and is represented by the symbol Z.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. Along with protons, they contribute to the atom's mass.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They are significantly lighter than protons and neutrons.
In a neutral atom, the number of protons equals the number of electrons, ensuring a balanced charge. This is a crucial concept for understanding isotopic variations.
What are Isotopes? Variations on a Theme
Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. This means they have the same atomic number (Z) but different mass numbers (A). The mass number (A) represents the total number of protons and neutrons in the nucleus.
Key Differences:
- Same Atomic Number (Z): Isotopes are variants of the same element, meaning they possess the same number of protons. This dictates their chemical properties.
- Different Mass Number (A): The difference lies in the number of neutrons, resulting in variations in mass. This affects their physical properties, such as density and radioactivity.
Example: Carbon Isotopes
Carbon (C) has an atomic number of 6, meaning all carbon atoms have 6 protons. However, carbon exists in several isotopic forms:
- Carbon-12 (¹²C): 6 protons + 6 neutrons = mass number 12 (most abundant isotope)
- Carbon-13 (¹³C): 6 protons + 7 neutrons = mass number 13 (stable isotope)
- Carbon-14 (¹⁴C): 6 protons + 8 neutrons = mass number 14 (radioactive isotope used in carbon dating)
How to Determine the Number of Subatomic Particles
The key to finding the number of protons, neutrons, and electrons lies in understanding the notation used to represent isotopes and utilizing simple formulas.
1. Isotope Notation:
Isotopes are typically represented using the following notation:
<sup>A</sup><sub>Z</sub>X
Where:
- A is the mass number (protons + neutrons)
- Z is the atomic number (number of protons)
- X is the element's chemical symbol
2. Calculating the Number of Subatomic Particles:
Using the isotope notation, we can easily calculate the number of each subatomic particle:
- Number of Protons (Z): This is directly given by the subscript in the isotope notation.
- Number of Neutrons: This is calculated by subtracting the atomic number (Z) from the mass number (A): Neutrons = A - Z
- Number of Electrons: In a neutral atom, the number of electrons is equal to the number of protons (Z). If the atom is an ion (charged), the number of electrons will be different, reflecting the ion's charge.
Example: Determining the Subatomic Particles in ¹⁴C
Let's apply this to Carbon-14 (¹⁴C):
-
Mass number (A): 14
-
Atomic number (Z): 6 (from the periodic table)
-
Number of Protons: 6
-
Number of Neutrons: 14 - 6 = 8
-
Number of Electrons (in a neutral atom): 6
Dealing with Ions: Charged Atoms
Remember, the above calculations apply to neutral atoms. Ions are atoms that have gained or lost electrons, resulting in a net electrical charge.
- Cations (Positive Ions): Atoms that have lost electrons. The number of electrons is less than the number of protons.
- Anions (Negative Ions): Atoms that have gained electrons. The number of electrons is greater than the number of protons.
To determine the number of electrons in an ion, you need to consider the ion's charge.
Example: Determining Subatomic Particles in ¹⁴C<sup>2-</sup>
Let's analyze the carbon-14 anion with a charge of -2 (¹⁴C<sup>2-</sup>):
-
Mass number (A): 14
-
Atomic number (Z): 6
-
Number of Protons: 6
-
Number of Neutrons: 14 - 6 = 8
-
Number of Electrons: 6 + 2 = 8 (two extra electrons due to the -2 charge)
Advanced Isotope Considerations: Abundance and Average Atomic Mass
Isotopes of an element often exist in nature with varying abundances. The average atomic mass listed on the periodic table is a weighted average of the masses of all isotopes, considering their relative abundances. This average mass is crucial in various chemical calculations.
The calculation of average atomic mass involves multiplying the mass of each isotope by its relative abundance (expressed as a decimal) and summing the results.
Practical Applications and Real-World Significance
Understanding isotopes is not merely an academic exercise; it has significant practical applications across numerous scientific fields:
- Nuclear Medicine: Radioactive isotopes are used in medical imaging (PET scans) and radiation therapy for cancer treatment.
- Carbon Dating: The radioactive isotope ¹⁴C is used to determine the age of ancient artifacts and organic materials.
- Geological Dating: Isotope ratios in rocks are used to estimate the age of geological formations and the Earth itself.
- Tracer Studies: Isotopes are used as tracers to follow the movement of atoms and molecules in biological and chemical systems.
- Nuclear Energy: Isotopes like Uranium-235 are crucial for nuclear power generation.
Troubleshooting Common Mistakes
While the process of determining protons, neutrons, and electrons in isotopes is relatively straightforward, some common errors can occur:
- Confusing Atomic Number and Mass Number: Ensure you correctly identify the atomic number (Z) and mass number (A) from the isotope notation.
- Ignoring Ion Charges: Remember to adjust the number of electrons if dealing with ions, adding or subtracting electrons to match the charge.
- Incorrect Calculation of Neutrons: Double-check your subtraction when calculating the number of neutrons (A - Z).
Conclusion: Mastering Isotope Calculations
This guide provides a comprehensive understanding of how to determine the number of protons, neutrons, and electrons in isotopes. By mastering these fundamental concepts and applying the techniques outlined, you will develop a strong foundation in atomic structure and be well-equipped to tackle more complex problems in chemistry and related fields. Remember to pay close attention to details, especially when dealing with ions and relative abundances, to avoid common calculation errors. The ability to accurately analyze isotopic composition opens doors to a deeper understanding of the world around us, from the ancient past to cutting-edge scientific advancements.
Latest Posts
Latest Posts
-
What Happens When Hydrate Is Heated
Apr 08, 2025
-
Axial Skeleton And Appendicular Skeleton Quiz
Apr 08, 2025
-
S And P Block Periodic Table
Apr 08, 2025
-
How To Subtract Fractions Mixed Numbers
Apr 08, 2025
-
What Are The Properties Of A Gas
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How To Find Protons Neutrons And Electrons Of Isotopes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.