How To Find Rate Constant K
Muz Play
Mar 22, 2025 · 6 min read
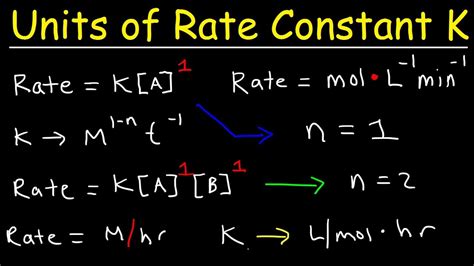
Table of Contents
How to Find the Rate Constant, k: A Comprehensive Guide
Determining the rate constant, k, is fundamental in chemical kinetics. It quantifies the speed of a chemical reaction, providing crucial insights into reaction mechanisms and predicting future behavior. This comprehensive guide delves into various methods for finding k, covering both theoretical underpinnings and practical applications. We will explore different reaction orders, data analysis techniques, and common pitfalls to avoid.
Understanding the Rate Law and Rate Constant
Before delving into methods for finding k, it's crucial to understand the rate law. The rate law expresses the relationship between the reaction rate and the concentrations of reactants. It generally takes the form:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>...
Where:
- Rate: The speed at which reactants are consumed or products are formed. Units are typically M/s (molarity per second) or mol L<sup>-1</sup> s<sup>-1</sup>.
- k: The rate constant. Its value is temperature-dependent and reflects the intrinsic reactivity of the system. The units of k depend on the overall reaction order (m + n + ...).
- [A], [B], ...: The molar concentrations of reactants A, B, etc.
- m, n, ...: The reaction orders with respect to reactants A, B, etc. These are experimentally determined and are not necessarily equal to the stoichiometric coefficients in the balanced chemical equation.
The overall reaction order is the sum of the individual reaction orders (m + n + ...). Common reaction orders include zero-order, first-order, second-order, and mixed-order reactions.
Determining the Reaction Order
Accurately determining the reaction order is paramount before calculating k. Several methods exist:
1. Method of Initial Rates
This method involves comparing the initial rates of reaction at different initial concentrations of reactants. By systematically varying the concentration of one reactant while keeping others constant, you can determine the order with respect to that reactant.
Example: Consider a reaction A + B → products.
If doubling [A] while keeping [B] constant doubles the initial rate, the reaction is first-order with respect to A. If doubling [A] quadruples the initial rate, it's second-order with respect to A. If doubling [A] has no effect on the initial rate, it's zero-order with respect to A. The same procedure is repeated for reactant B and any other reactants.
2. Graphical Method: Integrated Rate Laws
Each reaction order has a unique integrated rate law that relates concentration to time. Plotting the appropriate function of concentration versus time allows for determination of the reaction order and k.
- Zero-Order: [A]<sub>t</sub> = -kt + [A]<sub>0</sub>. A plot of [A]<sub>t</sub> vs. t yields a straight line with a slope of -k.
- First-Order: ln[A]<sub>t</sub> = -kt + ln[A]<sub>0</sub>. A plot of ln[A]<sub>t</sub> vs. t yields a straight line with a slope of -k.
- Second-Order: 1/[A]<sub>t</sub> = kt + 1/[A]<sub>0</sub>. A plot of 1/[A]<sub>t</sub> vs. t yields a straight line with a slope of k.
By plotting the appropriate function and observing linearity, you can confirm the reaction order and extract k from the slope.
3. Half-Life Method
The half-life (t<sub>1/2</sub>) is the time required for the concentration of a reactant to decrease to half its initial value. The relationship between half-life and k varies with reaction order:
- First-Order: t<sub>1/2</sub> = ln2/k
- Second-Order: t<sub>1/2</sub> = 1/(k[A]<sub>0</sub>)
By measuring the half-life at different initial concentrations (for second-order reactions) or simply measuring one half-life (for first-order reactions), you can calculate k.
Calculating the Rate Constant, k
Once the reaction order is determined, calculating k is straightforward using the appropriate integrated rate law or the initial rates method.
1. Using the Integrated Rate Laws
As shown above, the integrated rate laws provide a direct method for calculating k from the slope of a linear plot. The accuracy of k depends on the accuracy of the experimental data and the quality of the linear fit.
2. Using the Method of Initial Rates
This method relies on comparing initial rates at different concentrations. For example, if the reaction is first-order with respect to A and first-order with respect to B, the rate law is:
Rate = k[A][B]
By performing experiments with known initial concentrations and measuring the initial rate, you can solve for k:
k = Rate / ([A][B])
This requires multiple experiments to ensure accuracy and to account for experimental error.
Advanced Techniques and Considerations
1. Non-linear Regression Analysis
For reactions with complex rate laws or significant experimental error, linear regression might not be suitable. Non-linear regression techniques, available in software packages like Origin or Excel Solver, can fit the experimental data to the appropriate rate law and provide a more accurate estimate of k.
2. Temperature Dependence of k: Arrhenius Equation
The rate constant k is highly temperature-dependent. The Arrhenius equation describes this relationship:
k = Ae<sup>-Ea/RT</sup>
Where:
- A: The pre-exponential factor (frequency factor)
- Ea: The activation energy
- R: The gas constant
- T: The absolute temperature
By measuring k at different temperatures, you can plot ln(k) vs. 1/T to determine Ea and A. This allows you to predict k at other temperatures.
3. Parallel and Consecutive Reactions
For reactions involving multiple steps, the overall rate law can be more complex. Analysis of such reactions often requires more sophisticated techniques, possibly involving numerical methods for solving differential equations.
4. Catalysis
The presence of a catalyst significantly affects the rate of reaction and, consequently, the value of k. The rate law and k value will differ between catalyzed and uncatalyzed reactions.
Common Pitfalls and Error Analysis
- Incorrect Reaction Order: Misidentifying the reaction order leads to significant errors in k. Careful analysis of experimental data is crucial.
- Experimental Errors: Errors in concentration measurements, temperature control, and time measurements all contribute to uncertainty in k. Proper experimental design and error analysis are essential.
- Ignoring Side Reactions: If side reactions occur, the observed rate law might not accurately reflect the primary reaction, leading to inaccurate values of k.
- Non-ideal Conditions: Deviations from ideal conditions (e.g., non-ideal solutions, significant ionic strength) can affect reaction rates and k.
Conclusion
Determining the rate constant, k, is a crucial aspect of chemical kinetics. The choice of method depends on the reaction order and experimental conditions. Careful consideration of reaction order, data analysis techniques, and potential sources of error are essential for obtaining accurate and reliable values of k. Advanced techniques, like non-linear regression and the Arrhenius equation, allow for a more comprehensive understanding of reaction kinetics. Remember to always prioritize accurate experimental data and appropriate statistical analysis for reliable results. By mastering these methods, you gain invaluable insights into the fundamental processes governing chemical reactions.
Latest Posts
Latest Posts
-
What Is The Acceptable Macronutrient Distribution Range For Carbohydrates
Mar 23, 2025
-
How To Show Vectors Are Linearly Independent
Mar 23, 2025
-
An Equation Stating 2 Ratios Are Equal
Mar 23, 2025
-
During Glycolysis Glucose Is Broken Down Into Two Molecules Of
Mar 23, 2025
-
Ejemplos De Diagramas De Venn Resueltos
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Find Rate Constant K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
