How To Find Rate Of Diffusion
Muz Play
Mar 30, 2025 · 7 min read
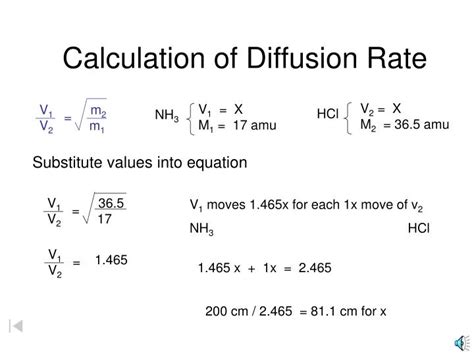
Table of Contents
How to Find the Rate of Diffusion: A Comprehensive Guide
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in various scientific fields. Understanding how to determine the rate of this movement is crucial for applications ranging from drug delivery to environmental science. This comprehensive guide delves into the methods and considerations involved in finding the rate of diffusion, covering both theoretical and practical aspects.
Understanding the Factors Affecting Diffusion Rate
Before we explore the methods for calculating the rate of diffusion, it's crucial to understand the factors that influence it. These factors directly impact the speed at which particles spread. Understanding these factors is key to designing accurate experiments and interpreting results correctly.
1. Concentration Gradient: The Driving Force
The concentration gradient, the difference in concentration between two regions, is the primary driving force behind diffusion. A steeper gradient (larger difference in concentration) results in a faster diffusion rate. Imagine dropping a dye into water – the dye spreads faster initially when the concentration difference is high and slows down as the dye becomes more evenly distributed.
2. Temperature: Molecular Kinetic Energy
Temperature plays a significant role. Higher temperatures mean molecules possess more kinetic energy, leading to increased movement and, consequently, a faster diffusion rate. The molecules are simply moving faster and colliding more frequently, leading to a more rapid dispersal.
3. Particle Size and Mass: Size Matters
The size and mass of the diffusing particles also affect the rate. Smaller and lighter particles diffuse faster than larger and heavier ones. This is because smaller particles experience less resistance as they move through a medium. Imagine trying to push a small marble versus a large bowling ball through a crowded room – the marble will navigate the obstacles more easily.
4. Medium: Viscosity and Permeability
The medium through which diffusion occurs significantly influences the rate. A less viscous (thinner) medium allows for faster diffusion than a more viscous (thicker) one. The permeability of the medium, its ability to allow substances to pass through, is also vital. A porous membrane will permit faster diffusion than a dense, impermeable barrier.
5. Distance: The Longer the Journey, the Slower the Pace
The distance over which diffusion must occur is another critical factor. The greater the distance, the slower the diffusion rate. This is because particles have to travel further to reach equilibrium. Think of spreading a scent across a small room versus a large hall – the scent will reach the far corners more quickly in the smaller room.
Methods for Determining the Rate of Diffusion
Several methods exist for determining the rate of diffusion, each with its advantages and limitations. The choice of method depends on the specific system being studied and the available resources.
1. Fick's First Law of Diffusion: A Theoretical Approach
Fick's First Law provides a theoretical framework for understanding and quantifying diffusion. It states that the rate of diffusion (J) is proportional to the concentration gradient (dC/dx):
J = -D (dC/dx)
Where:
- J represents the flux (amount of substance diffusing per unit area per unit time).
- D is the diffusion coefficient, a constant that depends on the properties of the diffusing substance and the medium.
- dC/dx is the concentration gradient, the change in concentration over the change in distance. The negative sign indicates that diffusion occurs down the concentration gradient (from high to low concentration).
Determining D (the diffusion coefficient): The diffusion coefficient, D, isn't directly measurable in many scenarios. Instead, it often needs to be determined experimentally or obtained from literature for specific systems under defined conditions. The value of D can vary significantly depending on temperature, the nature of the diffusing substance, and the medium.
2. Experimental Methods: Measuring Diffusion in Action
Several experimental techniques allow for the direct measurement of diffusion rates. These methods provide practical, real-world data that can complement theoretical calculations.
a) The Diffusion Cell Method: A Controlled Environment
A diffusion cell is a device designed to control and measure diffusion across a membrane or barrier. A known concentration of the substance is placed on one side of the membrane, and the change in concentration on the other side is monitored over time. The rate of diffusion can then be calculated based on this change in concentration. Different cell designs cater to specific needs—some employ stirring to maintain homogeneity, while others use specialized membranes to control permeability.
b) Tracer Techniques: Tracking Diffusion
Tracer techniques utilize radioactively labeled or fluorescently tagged molecules to track their movement during diffusion. The movement of these tagged molecules can be observed over time using various techniques such as chromatography, spectroscopy, or imaging. This method is particularly useful for studying diffusion in complex systems where direct concentration measurements might be challenging.
c) Microscopic Techniques: Observing Diffusion at the Microscopic Level
Advanced microscopy techniques, such as fluorescence recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy (FCS), allow for the observation of diffusion at a microscopic level. These methods provide information on diffusion coefficients and other dynamic parameters with high spatial and temporal resolution.
3. Computational Methods: Simulating Diffusion
For complex systems where experimental measurement is difficult or impossible, computational methods such as molecular dynamics simulations can be used to simulate diffusion processes. These simulations provide valuable insights into the diffusion mechanisms at the molecular level and can be used to predict diffusion rates under various conditions. These simulations, however, require significant computational power and expertise.
Applications of Diffusion Rate Determination
Determining the rate of diffusion has far-reaching applications across various scientific and engineering disciplines. Here are some key examples:
-
Pharmacokinetics and Drug Delivery: Understanding how drugs diffuse through biological tissues is crucial for designing effective drug delivery systems. Diffusion rate measurements help determine optimal drug formulations and administration routes.
-
Environmental Science: Diffusion plays a significant role in the transport of pollutants in soil and water. Determining diffusion rates helps model contaminant spread and develop remediation strategies.
-
Material Science: Diffusion processes are essential in various material synthesis and processing techniques. Understanding diffusion rates is crucial for controlling material properties and ensuring product quality.
-
Food Science: Diffusion affects the flavor and texture of foods during processing and storage. Measuring diffusion rates can help optimize processing techniques and extend shelf life.
-
Biology: Diffusion is fundamental to many biological processes, including nutrient transport, waste removal, and signal transduction. Studying diffusion rates aids in understanding various cellular and physiological processes.
Challenges and Considerations
While determining the rate of diffusion can be done using various methods, it's important to be aware of potential challenges and limitations:
-
Non-ideal systems: Fick's First Law assumes ideal conditions, such as constant temperature, uniform medium properties, and no interactions between diffusing particles. In real-world systems, these conditions might not always be met, leading to deviations from the expected diffusion rates.
-
Experimental errors: Experimental measurements are always susceptible to errors. Careful experimental design, precise measurements, and appropriate data analysis are essential to minimize errors and ensure the accuracy of results.
-
Complex systems: In complex systems such as biological tissues, the diffusion process can be influenced by multiple factors and pathways, making it challenging to accurately determine diffusion rates.
Conclusion
Determining the rate of diffusion is a complex yet vital task with broad applications. Understanding the factors that influence diffusion, choosing the appropriate methodology based on the specific system under investigation, and acknowledging the potential limitations are crucial for obtaining accurate and meaningful results. This comprehensive guide serves as a foundation for researchers, students, and professionals alike who need to understand and measure diffusion rates in their respective fields. The combination of theoretical frameworks, experimental techniques, and computational simulations provides a powerful toolkit for tackling the challenges of diffusion research. By employing these methods and understanding their nuances, we can unlock the secrets of diffusion and harness its power for various applications.
Latest Posts
Latest Posts
-
Structures 1 2 And 3 Make Up A
Apr 01, 2025
-
What Is The Biocultural Approach In Biological Anthropology
Apr 01, 2025
-
Multiplying And Dividing Sig Figs Practice
Apr 01, 2025
-
Find An Equation For The Tangent Plane To The Surface
Apr 01, 2025
-
All Regions Of Earth Where Organisms Live
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Find Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
