How To Find The Equivalence Point Of A Titration
Muz Play
Mar 17, 2025 · 6 min read
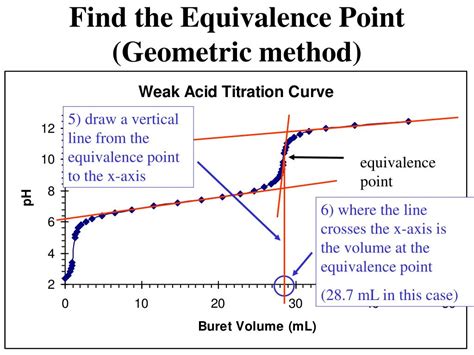
Table of Contents
How to Find the Equivalence Point of a Titration: A Comprehensive Guide
Titration, a cornerstone technique in analytical chemistry, is used to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). The equivalence point, the crucial point in a titration, represents the exact moment when the moles of titrant added equal the moles of analyte present. Accurately determining this point is vital for obtaining accurate results. This comprehensive guide will delve into various methods for finding the equivalence point, including visual indicators, instrumental methods, and data analysis techniques.
Understanding the Equivalence Point and its Significance
Before diving into the methods, let's solidify our understanding of the equivalence point. It's the stoichiometric point in a titration where the added titrant has completely reacted with the analyte. This doesn't necessarily mean the pH is 7; it simply signifies the complete neutralization or reaction. The equivalence point is a theoretical concept, while the endpoint, which we observe experimentally, is an approximation. The difference between these two points is known as the titration error.
Why is accurately determining the equivalence point so crucial?
- Accurate Concentration Determination: The primary goal of a titration is to determine the concentration of the unknown solution. The equivalence point provides the precise data needed for this calculation.
- Stoichiometric Calculations: Knowing the equivalence point allows for precise stoichiometric calculations related to the reaction, providing insights into reaction ratios and molar masses.
- Understanding Reaction Kinetics: The equivalence point can offer clues about the kinetics of the reaction, particularly if the reaction rate is slow or complex.
- Quality Control: In various industries, titrations are used for quality control purposes. Accurate determination of the equivalence point ensures the product meets required specifications.
Methods for Finding the Equivalence Point
Several methods exist for determining the equivalence point, each with its strengths and limitations:
1. Visual Indicators: A Classic Approach
Visual indicators are substances that change color near the equivalence point. These indicators are weak acids or bases that exhibit different colors in their acidic and basic forms. The color change signifies a significant change in pH, indicating proximity to the equivalence point.
Choosing the Right Indicator: The selection of an appropriate indicator is crucial. The indicator's pKa (acid dissociation constant) should be close to the pH at the equivalence point. A suitable indicator will change color within the pH range of the steep portion of the titration curve near the equivalence point. For instance:
- Strong Acid-Strong Base Titration: Phenolphthalein (colorless to pink) or methyl orange (red to yellow) are common indicators.
- Weak Acid-Strong Base Titration: Phenolphthalein is often preferred because the equivalence point pH is greater than 7.
- Weak Base-Strong Acid Titration: Methyl orange is frequently chosen because the equivalence point pH is less than 7.
Limitations of Visual Indicators:
- Subjectivity: The color change perception can be subjective, leading to slight variations in the endpoint determination.
- Indicator Error: The endpoint may not precisely coincide with the equivalence point, introducing an indicator error.
- Not Suitable for all Titrations: Some titrations lack a significant pH change near the equivalence point, making visual indicators unsuitable.
2. Instrumental Methods: Precise and Objective
Instrumental methods offer a more precise and objective approach to equivalence point determination. These methods rely on continuously monitoring a physical property of the solution during the titration, producing a titration curve. The equivalence point is identified from the inflection point or a sharp change in the curve.
Common Instrumental Methods:
- pH Meter: This is the most prevalent instrumental method. A pH electrode continuously measures the solution's pH as the titrant is added. The equivalence point is identified from the sharpest change in pH on the titration curve.
- Conductivity Meter: Conductivity measurements track changes in the solution's electrical conductivity. The equivalence point is usually marked by a sharp change in conductivity.
- Spectrophotometry: This method uses light absorption to monitor the concentration of reactants or products. The equivalence point is identified from the change in absorbance.
- Potentiometry: This technique measures the potential difference between two electrodes. It’s particularly useful for redox titrations.
Advantages of Instrumental Methods:
- Objective Measurement: Removes subjectivity associated with visual indicators.
- Higher Precision: Provides more accurate determination of the equivalence point.
- Suitable for Various Titrations: Can be used for titrations where visual indicators are ineffective.
3. Data Analysis Techniques: Extracting Information from Curves
Once a titration curve (either from instrumental methods or carefully plotted data from visual observations) is obtained, several data analysis techniques can help pinpoint the equivalence point:
- First Derivative Method: The first derivative of the titration curve (ΔpH/ΔV or ΔConductivity/ΔV) is calculated. The equivalence point is located at the maximum of the first derivative curve.
- Second Derivative Method: The second derivative (Δ(ΔpH/ΔV)/ΔV or Δ(ΔConductivity/ΔV)/ΔV) provides even greater precision. The equivalence point is at the zero-crossing point of the second derivative curve.
- Gran Plot: This method is particularly useful for titrations involving weak acids or bases. It involves plotting a function of the measured pH or conductivity against the titrant volume. The equivalence point is determined by extrapolating the linear portion of the plot.
- Software Analysis: Many software packages are available for analyzing titration data, automating the process of determining the equivalence point. These programs often incorporate multiple methods, including derivative analysis and Gran plots, providing robust and reliable results.
Practical Considerations and Troubleshooting
While the methods outlined above provide a comprehensive approach, several practical considerations influence the accuracy of equivalence point determination:
- Solution Purity: Ensuring the purity of both the analyte and titrant is essential. Impurities can lead to inaccurate results.
- Proper Calibration: Calibration of instruments (pH meters, conductivity meters) is critical for reliable measurements.
- Appropriate Titration Rate: A slow and steady addition of titrant near the equivalence point enhances accuracy. Too rapid addition can lead to overshooting the endpoint.
- Temperature Control: Maintaining a constant temperature throughout the titration is important as temperature changes can affect the equilibrium of the reaction and potentially the indicator's color change.
- Avoiding Air Exposure: For some titrations, air exposure might interfere with the reaction. Taking steps to minimize air exposure is advisable.
- Proper Mixing: Thorough mixing of the solution during titration ensures complete reaction between the titrant and analyte.
Troubleshooting Common Issues:
- Over-shooting the Endpoint: Slow down the titrant addition near the endpoint. Use smaller increments of titrant as the endpoint is approached.
- Unclear Endpoint: If the endpoint is unclear, try a different indicator, use an instrumental method, or ensure the solution is properly mixed.
- Inaccurate Results: Double-check the purity of solutions, the calibration of instruments, and the calculation procedure.
Conclusion
Determining the equivalence point in a titration is paramount for achieving accurate and reliable results. Whether employing visual indicators, sophisticated instrumental methods, or data analysis techniques, the choice of method depends on the specific titration, the desired accuracy, and the available resources. By carefully considering the strengths and limitations of each method and addressing practical considerations, chemists and scientists can confidently and precisely determine the equivalence point, opening the door to accurate quantitative analysis in various applications. This precise determination is crucial for a wide range of fields, from environmental monitoring and pharmaceutical quality control to research and development in various scientific disciplines. Understanding the nuances of each method allows for effective problem-solving and the generation of high-quality data.
Latest Posts
Latest Posts
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Equivalence Point Of A Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
