How To Identify A Weak Acid
Muz Play
Mar 15, 2025 · 6 min read
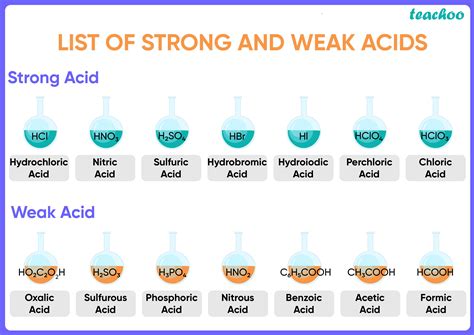
Table of Contents
How to Identify a Weak Acid: A Comprehensive Guide
Identifying a weak acid requires a multifaceted approach combining theoretical understanding with practical experimentation. This guide will delve into the various methods used to determine whether a substance is a weak acid, exploring the underlying principles and offering practical advice. We will cover theoretical considerations, laboratory tests, and the interpretation of results, providing a comprehensive understanding of this important topic in chemistry.
Understanding the Definition of a Weak Acid
Before delving into identification methods, it's crucial to understand what constitutes a weak acid. A weak acid is an acid that only partially dissociates (ionizes) in a solution. This means that only a small fraction of the acid molecules break down into their constituent ions (H⁺ and an anion) when dissolved in water. This contrasts sharply with strong acids, which completely dissociate in solution. The extent of dissociation is quantified by the acid dissociation constant (Ka). A weak acid has a Ka value significantly less than 1, typically ranging from 10⁻² to 10⁻¹⁴. The smaller the Ka value, the weaker the acid.
Key Differences Between Weak and Strong Acids
| Feature | Weak Acid | Strong Acid |
|---|---|---|
| Dissociation | Partial dissociation in solution | Complete dissociation in solution |
| Ka Value | Ka << 1 (typically 10⁻² to 10⁻¹⁴) | Ka >> 1 (typically > 1) |
| pH of Solution | Higher pH for a given concentration | Lower pH for a given concentration |
| Conductivity | Lower electrical conductivity | Higher electrical conductivity |
| Examples | Acetic acid (CH₃COOH), carbonic acid (H₂CO₃), hydrofluoric acid (HF) | Hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃) |
Methods for Identifying a Weak Acid
Identifying a weak acid involves a combination of theoretical knowledge and practical experimentation. Let's explore the various approaches:
1. Examining the Chemical Formula and Structure
While not definitive on its own, the chemical formula and structure of a compound can provide clues. Certain functional groups are strongly associated with weak acidity. These include:
- Carboxylic acids (-COOH): These are arguably the most common weak acid functional group, found in organic acids like acetic acid and citric acid. The presence of a carboxyl group is a strong indicator of weak acidity.
- Phenols (-OH attached to an aromatic ring): Phenols are weakly acidic due to the resonance stabilization of the phenoxide ion.
- Thiols (-SH): Similar to alcohols, thiols exhibit weak acidity, though generally weaker than carboxylic acids or phenols.
Note: The presence of these functional groups doesn't guarantee weak acidity; the surrounding molecular structure significantly influences the acidity.
2. Measuring pH
The pH of a solution provides a direct indication of the acidity. A pH meter is the most accurate way to measure the pH. Weak acids will have a pH higher than strong acids at the same concentration. However, a high pH alone is not sufficient to definitively classify a substance as a weak acid. Other factors like concentration must be considered.
3. Determining the Acid Dissociation Constant (Ka)
The most definitive way to identify a weak acid is by determining its Ka value experimentally. This involves measuring the concentration of the acid and its conjugate base in solution and applying the following equation:
Ka = [H⁺][A⁻] / [HA]
Where:
- [H⁺] is the concentration of hydrogen ions.
- [A⁻] is the concentration of the conjugate base.
- [HA] is the concentration of the undissociated acid.
Determining Ka often involves techniques like pH titration. By titrating the unknown acid with a strong base of known concentration, one can plot a titration curve. The Ka value can then be calculated from the half-equivalence point of the titration curve, where [HA] = [A⁻]. This method allows for precise determination of Ka and, consequently, the confirmation of weak acidity.
4. Conductivity Measurement
Weak acids are poor conductors of electricity compared to strong acids. This is because they only partially dissociate, resulting in a lower concentration of charge carriers (ions) in the solution. A conductivity meter can measure the electrical conductivity of the solution. Lower conductivity suggests weak acidity. However, this is a less precise method than measuring Ka.
5. Spectroscopic Techniques
Certain spectroscopic techniques, like Infrared (IR) spectroscopy and Nuclear Magnetic Resonance (NMR) spectroscopy, can provide structural information supporting the identification. For example, IR spectroscopy can help identify the presence of functional groups associated with weak acids, such as the carboxyl group in carboxylic acids. NMR spectroscopy can help determine the molecular environment of the acidic proton, providing further insight into the acidity strength. However, these techniques alone are insufficient to definitively identify a weak acid; they must be complemented by other experimental techniques.
6. Chemical Reactions and Observations
Certain chemical reactions can indicate weak acidity. Reactions with carbonates or bicarbonates, for instance, produce carbon dioxide gas if the acid is strong enough to overcome the carbonate's buffering capacity. The rate of gas evolution can give an indication of the acid's strength. A slow gas evolution might suggest weak acidity. However, this method is qualitative and not precise for determining the strength of the acid.
Practical Considerations and Interpretation of Results
When identifying a weak acid, several practical aspects are crucial:
- Purity of the sample: Impurities can significantly affect the experimental results. It is essential to use a pure sample to obtain reliable data.
- Temperature control: Temperature impacts the equilibrium of acid dissociation, and consistent temperature control is vital for accurate measurements.
- Appropriate concentration: The chosen concentration of the acid must be suitable for the employed experimental technique. Too concentrated, and the measurements might be outside the range of the instrument; too dilute, and the measurements might be less accurate.
- Accuracy of measurements: Employing appropriate measuring devices and careful experimental techniques are crucial for accuracy and reliability.
The interpretation of the results involves combining the data from different techniques. A low Ka value, high pH for a given concentration, low conductivity, and the presence of common weak acid functional groups strongly suggest a weak acid. However, relying on a single method is not recommended; multiple lines of evidence are necessary for a conclusive identification.
Examples of Weak Acids and their Identification
Let's consider a few examples:
1. Acetic Acid (CH₃COOH): This is a classic example of a weak acid. Its Ka value is approximately 1.8 x 10⁻⁵. Its pH is relatively higher than a strong acid at the same concentration. IR spectroscopy would show the characteristic peaks of the carboxyl group.
2. Carbonic Acid (H₂CO₃): This diprotic acid is a weak acid, crucial in biological systems. Its Ka values are relatively low, reflecting its weak acidity. The observation of CO₂ gas upon reaction with a carbonate would support its acidic nature.
3. Hydrofluoric Acid (HF): While classified as a weak acid, it's a relatively stronger weak acid than acetic acid or carbonic acid, having a Ka value approximately 7.2 x 10⁻⁴. It's important to note that classifying an acid as "weak" doesn't imply an insignificant level of acidity; it simply indicates incomplete dissociation.
Conclusion
Identifying a weak acid is a process involving careful experimentation and interpretation. Multiple techniques are necessary for a confident identification, including pH measurement, Ka determination via titration, conductivity measurement, and potentially spectroscopic analysis. Understanding the theoretical underpinnings of weak acids, their characteristic properties, and the strengths and limitations of different identification methods is paramount to accurately classify a substance as a weak acid. Remember always to prioritize safety and use appropriate laboratory techniques when performing experiments.
Latest Posts
Latest Posts
-
What Is The Path Of A Projectile Called
May 09, 2025
-
The First Movement Of The Spring Concerto Is Programmatic
May 09, 2025
-
List Three Rules To Remember When Focusing A Microscope
May 09, 2025
-
In Eukaryotic Cells Dna Is Found In The
May 09, 2025
-
Bond Order Of No In No3
May 09, 2025
Related Post
Thank you for visiting our website which covers about How To Identify A Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.