How To Recognize A Redox Reaction
Muz Play
Mar 20, 2025 · 6 min read
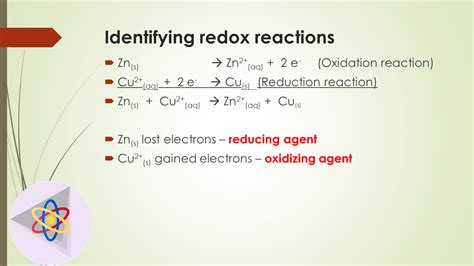
Table of Contents
How to Recognize a Redox Reaction: A Comprehensive Guide
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin a vast array of natural phenomena and industrial applications. From rusting iron to photosynthesis, the transfer of electrons lies at the heart of these reactions. Recognizing a redox reaction, however, can sometimes be challenging. This comprehensive guide will equip you with the knowledge and tools to confidently identify redox reactions in various contexts.
Understanding the Basics: Oxidation and Reduction
Before delving into the recognition of redox reactions, it's crucial to grasp the core concepts of oxidation and reduction. These two processes are inseparable, always occurring simultaneously.
Oxidation: Loss of Electrons
Oxidation is defined as the loss of electrons by an atom, ion, or molecule. This loss results in an increase in the oxidation state of the species involved. Remember the mnemonic OIL RIG – Oxidation Is Loss, Reduction Is Gain (of electrons).
Examples of Oxidation:
- The formation of iron(III) oxide (rust): Iron atoms lose electrons to oxygen atoms, forming Fe³⁺ ions and oxidizing the iron.
- The combustion of methane: Carbon atoms in methane (CH₄) lose electrons to oxygen atoms during combustion, resulting in the formation of carbon dioxide (CO₂) and water (H₂O).
- The reaction of magnesium with hydrochloric acid: Magnesium atoms lose electrons to hydrogen ions (H⁺), forming magnesium ions (Mg²⁺) and hydrogen gas (H₂).
Reduction: Gain of Electrons
Reduction is the gain of electrons by an atom, ion, or molecule. This gain leads to a decrease in the oxidation state.
Examples of Reduction:
- The formation of iron(III) oxide (rust): Oxygen atoms gain electrons from iron atoms, forming O²⁻ ions and reducing the oxygen.
- The formation of copper metal from copper(II) ions: Copper(II) ions (Cu²⁺) gain electrons, becoming neutral copper atoms (Cu).
- The reduction of nitrate ions to nitrite ions: Nitrate ions (NO₃⁻) gain electrons, forming nitrite ions (NO₂⁻).
Key Indicators of Redox Reactions
Several key indicators can help you identify a redox reaction. Let's explore these indicators in detail:
1. Change in Oxidation States
The most definitive way to identify a redox reaction is by observing a change in the oxidation states of the elements involved. You need to assign oxidation states to each element in the reactants and products. If at least one element's oxidation state changes, it's a redox reaction.
Assigning Oxidation States: Several rules govern oxidation state assignment:
- The oxidation state of an element in its elemental form is always 0. (e.g., O₂, Cl₂, Na)
- The oxidation state of a monatomic ion is equal to its charge. (e.g., Na⁺ = +1, Cl⁻ = -1)
- The oxidation state of hydrogen is usually +1, except in metal hydrides where it's -1.
- The oxidation state of oxygen is usually -2, except in peroxides (-1) and superoxides (-1/2).
- The sum of oxidation states in a neutral molecule is 0.
- The sum of oxidation states in a polyatomic ion equals the charge of the ion.
Example: Consider the reaction between zinc and copper(II) sulfate:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
- Reactants: Zn (oxidation state = 0), Cu (oxidation state = +2), S (+6), O (-2)
- Products: Zn (oxidation state = +2), Cu (oxidation state = 0), S (+6), O (-2)
Notice that zinc's oxidation state increased from 0 to +2 (oxidation), while copper's oxidation state decreased from +2 to 0 (reduction). This change in oxidation states confirms that this is a redox reaction.
2. Presence of Oxidizing and Reducing Agents
Redox reactions involve two key players: oxidizing agents and reducing agents.
-
Oxidizing agents are substances that cause oxidation by accepting electrons. They themselves are reduced in the process. Common oxidizing agents include oxygen (O₂), potassium permanganate (KMnO₄), and potassium dichromate (K₂Cr₂O₇).
-
Reducing agents are substances that cause reduction by donating electrons. They themselves are oxidized in the process. Common reducing agents include metals (like zinc and magnesium), and certain organic compounds.
Identifying the presence of a strong oxidizing or reducing agent often suggests a redox reaction.
3. Transfer of Electrons
The fundamental characteristic of a redox reaction is the transfer of electrons from one species to another. While not always directly observable, the changes in oxidation states directly reflect this electron transfer. In some reactions, like those involving electrode processes, the electron transfer is directly involved in the mechanism.
4. Common Redox Reaction Patterns
Recognizing certain common reaction patterns can help in identifying redox reactions:
- Combustion reactions: Reactions involving rapid oxidation of a substance, typically with oxygen, releasing heat and light.
- Corrosion: The gradual oxidation of metals, such as the rusting of iron.
- Single displacement reactions: Reactions where one element replaces another in a compound. (e.g., Zn + CuSO₄ → ZnSO₄ + Cu)
- Disproportionation reactions: Reactions where the same element is both oxidized and reduced. (e.g., 2H₂O₂ → 2H₂O + O₂)
Advanced Techniques for Redox Reaction Identification
For more complex reactions, additional techniques can be employed:
1. Half-Reaction Method
This method involves separating the overall redox reaction into two half-reactions: an oxidation half-reaction and a reduction half-reaction. Balancing these half-reactions individually and then combining them can help confirm the redox nature of the overall reaction.
2. Using Standard Reduction Potentials
Standard reduction potentials (E°) are a measure of the tendency of a species to gain electrons. A more positive E° indicates a greater tendency to be reduced. Comparing the reduction potentials of the reactants can predict the spontaneity of a redox reaction and confirm its occurrence.
Common Mistakes to Avoid
Several common mistakes can lead to misidentification of redox reactions:
- Focusing solely on oxygen: While oxygen often participates in redox reactions, its presence doesn't automatically indicate a redox reaction. Many reactions involve electron transfer without oxygen participation.
- Ignoring changes in oxidation states of less common elements: Always consider all elements present, not just the readily apparent ones.
- Confusing acid-base reactions with redox reactions: Acid-base reactions involve proton transfer, not electron transfer. Do not confuse these two distinct reaction types.
Conclusion
Recognizing redox reactions requires a clear understanding of oxidation and reduction, the ability to assign oxidation states, and the awareness of key indicators such as changes in oxidation states, the presence of oxidizing and reducing agents, and the transfer of electrons. By mastering these concepts and applying the techniques discussed in this guide, you'll be well-equipped to confidently identify redox reactions in a variety of chemical contexts. Remember to practice regularly with different examples to solidify your understanding and skill in recognizing these fundamental chemical processes. The more you practice, the easier it will become to spot the subtle yet crucial signs of electron transfer.
Latest Posts
Latest Posts
-
The Mean Of The Sample Means
Mar 21, 2025
-
Is Sulfur More Electronegative Than Oxygen
Mar 21, 2025
-
Color The Neuron And Neuroglial Cells Answer Key
Mar 21, 2025
-
The Basic Unit Of Life Is The
Mar 21, 2025
-
Calorimetry And Hesss Law Pre Lab Answers
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How To Recognize A Redox Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
