How To Tell The Difference Between Ionic And Molecular Compounds
Muz Play
Mar 19, 2025 · 6 min read
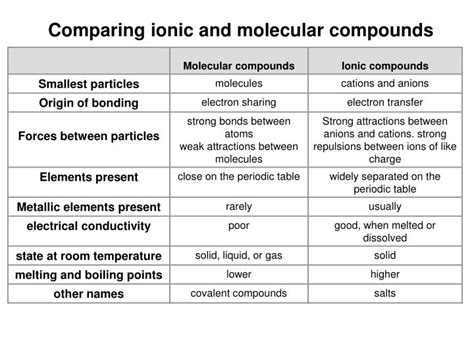
Table of Contents
How to Tell the Difference Between Ionic and Molecular Compounds
Understanding the difference between ionic and molecular compounds is fundamental to grasping the principles of chemistry. These two types of compounds exhibit vastly different properties, stemming from the nature of the bonds holding their constituent atoms together. This comprehensive guide will equip you with the knowledge to confidently distinguish between them, exploring various identification methods and providing illustrative examples.
The Fundamental Difference: Bond Type
At the heart of the distinction lies the type of chemical bond present.
-
Ionic compounds are formed through the transfer of electrons from a metal to a nonmetal. This transfer creates ions: positively charged cations (metals) and negatively charged anions (nonmetals). The electrostatic attraction between these oppositely charged ions forms the ionic bond, a strong electrostatic force.
-
Molecular compounds, also known as covalent compounds, are formed when atoms share electrons. This sharing occurs typically between nonmetals, resulting in a covalent bond. The shared electrons create a relatively strong attraction holding the atoms together within a molecule.
This fundamental difference in bonding mechanisms leads to a cascade of differing physical and chemical properties.
Identifying Ionic Compounds: Key Characteristics
Several characteristics reliably indicate the presence of an ionic compound. Learning to recognize these features is crucial for accurate identification.
1. Composition: Metal and Nonmetal
The simplest and often most reliable indicator is the composition of the compound. Ionic compounds invariably consist of a metal combined with a nonmetal. Metals readily lose electrons (low electronegativity), while nonmetals readily gain electrons (high electronegativity). This difference in electronegativity drives the electron transfer that defines ionic bonding.
Examples:
- NaCl (Sodium Chloride): Sodium (Na), an alkali metal, loses an electron to chlorine (Cl), a halogen.
- MgO (Magnesium Oxide): Magnesium (Mg), an alkaline earth metal, loses two electrons to oxygen (O), a chalcogen.
- Al₂O₃ (Aluminum Oxide): Aluminum (Al), a post-transition metal, loses three electrons per atom to oxygen.
2. High Melting and Boiling Points
Ionic compounds generally possess high melting and boiling points. This is a direct consequence of the strong electrostatic forces between the ions. A significant amount of energy is required to overcome these attractions and break apart the crystal lattice structure characteristic of ionic solids.
Comparison: Compare the melting point of NaCl (801 °C) with that of a molecular compound like water (0 °C). The vast difference highlights the strength of ionic bonds.
3. Crystalline Structure
Ionic compounds typically exist as crystalline solids at room temperature. These crystals are characterized by a highly ordered, three-dimensional arrangement of ions, maximizing electrostatic attraction and minimizing repulsion. The regular arrangement of ions contributes to the characteristic shapes of ionic crystals.
4. Conductivity: When Dissolved or Molten
Ionic compounds are generally poor conductors of electricity in their solid state. However, they become excellent conductors when dissolved in water or molten. This is because the ions are free to move and carry an electric current when not locked in a rigid crystal lattice.
5. Brittleness
Ionic crystals are usually brittle and tend to shatter under stress. This is because the application of force can misalign the layers of ions, causing repulsive forces between ions of like charge to dominate, leading to fracture.
Identifying Molecular Compounds: Distinguishing Features
Molecular compounds exhibit a contrasting set of properties compared to ionic compounds, stemming from the nature of their covalent bonds.
1. Composition: Nonmetals Only
The key compositional feature of molecular compounds is the involvement of only nonmetals. Since covalent bonds involve electron sharing between atoms with similar electronegativities, the constituent atoms are typically nonmetals.
Examples:
- H₂O (Water): Two hydrogen atoms share electrons with an oxygen atom.
- CO₂ (Carbon Dioxide): Carbon shares electrons with two oxygen atoms.
- CH₄ (Methane): Carbon shares electrons with four hydrogen atoms.
2. Lower Melting and Boiling Points
Molecular compounds generally have lower melting and boiling points compared to ionic compounds. The intermolecular forces (forces between molecules) holding molecular compounds together are weaker than the electrostatic forces in ionic compounds. Therefore, less energy is required to overcome these forces and change the state of matter.
3. Variable Physical States
Molecular compounds can exist in various states at room temperature – solids, liquids, or gases. This diversity reflects the range of intermolecular forces present and their strength relative to thermal energy.
4. Poor Conductivity
Molecular compounds are generally poor conductors of electricity in all states (solid, liquid, or gas). This is because they do not contain freely moving charged particles (ions) to carry an electric current. Some exceptions exist for highly polar molecules in solution that can slightly dissociate and contribute some conductivity.
5. Often Flexible or Soft
Unlike the brittleness of ionic compounds, many molecular compounds are flexible or soft. This arises from the weaker intermolecular forces and the absence of a rigid crystalline structure like ionic compounds.
Ambiguity and Exceptions: Understanding the Gray Areas
While the distinctions outlined above are generally reliable, there are instances where the lines blur. Some compounds may exhibit properties that seem to fall between the typical characteristics of purely ionic or purely covalent compounds.
Polar Covalent Compounds
Polar covalent compounds represent an intermediate case. They involve covalent bonds but the atoms involved have differing electronegativities, resulting in an uneven distribution of electron density. This creates a dipole moment, giving the molecule a partial positive and partial negative end. These compounds can exhibit some properties that are partially ionic in nature, such as higher boiling points than non-polar molecular compounds.
Polyatomic Ions
The presence of polyatomic ions adds another layer of complexity. These ions are groups of atoms covalently bonded together that carry a net charge. These ions participate in ionic bonding with other ions to form ionic compounds. For example, in ammonium nitrate (NH₄NO₃), the ammonium ion (NH₄⁺) and nitrate ion (NO₃⁻) are covalently bonded internally but are ionically bonded to each other.
Applying the Knowledge: Practical Examples
Let's test your understanding with some examples:
1. KBr (Potassium Bromide): This compound contains potassium (a metal) and bromine (a nonmetal). It will have a high melting point, be a crystalline solid, and be a good conductor of electricity when molten or dissolved. It is an ionic compound.
2. CO (Carbon Monoxide): This compound consists only of nonmetals (carbon and oxygen). It will have a low melting point, likely exist as a gas at room temperature, and be a poor conductor of electricity. It is a molecular compound.
3. (NH₄)₂SO₄ (Ammonium Sulfate): This contains the polyatomic ions ammonium (NH₄⁺) and sulfate (SO₄²⁻). While the internal bonds within the polyatomic ions are covalent, the overall bonding in the compound is ionic due to the electrostatic attraction between the polyatomic ions. It behaves primarily as an ionic compound.
4. CH₃COOH (Acetic Acid): This is a molecular compound composed entirely of nonmetals (carbon, hydrogen, and oxygen). It's a liquid at room temperature, has a relatively low melting point, and is a poor conductor of electricity. It is a molecular compound.
Conclusion: Mastering the Identification of Compound Types
Distinguishing between ionic and molecular compounds is a cornerstone of chemical understanding. By carefully analyzing the composition, physical properties, and electrical conductivity of a compound, one can reliably categorize it. Remember that while the general rules are clear, nuances and exceptions exist, particularly with polar covalent compounds and those containing polyatomic ions. A deeper understanding of chemical bonding and intermolecular forces solidifies the ability to accurately differentiate between these fundamental compound types. Practice is key – the more examples you analyze, the more adept you will become at this essential skill.
Latest Posts
Latest Posts
-
Does A Plant Cell Have Endoplasmic Reticulum
Mar 19, 2025
-
How To Add Rational Algebraic Expressions
Mar 19, 2025
-
Phase Addition Subtraction Multiplication And Division
Mar 19, 2025
-
A First Course In Differential Equations
Mar 19, 2025
-
What Does Poly Mean Before A Chemical Compound
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Tell The Difference Between Ionic And Molecular Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
