Identify The Chirality Center In Each Molecule
Muz Play
Apr 07, 2025 · 5 min read
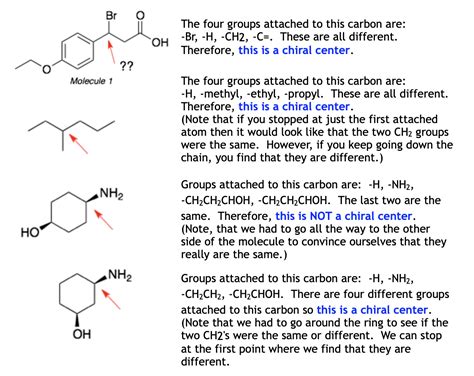
Table of Contents
Identifying Chirality Centers in Molecules: A Comprehensive Guide
Chirality, a fundamental concept in organic chemistry, describes the spatial arrangement of atoms in a molecule. A molecule is chiral if it is not superimposable on its mirror image. This lack of superimposable mirror images is known as optical isomerism or enantiomerism. The key to identifying chirality often lies in recognizing chirality centers, also known as stereocenters or asymmetric carbons. This comprehensive guide will delve into the intricacies of identifying chirality centers in various molecules, providing you with the knowledge and tools to master this crucial aspect of organic chemistry.
Understanding Chirality and Chirality Centers
Before we delve into identification, let's solidify our understanding of the core concepts. A chirality center is typically a carbon atom bonded to four different groups. This tetrahedral arrangement creates two non-superimposable mirror images, called enantiomers. It's crucial to remember that the four groups must be distinct; if any two groups are identical, the carbon atom is not a chirality center.
Identifying a Chirality Center: The Four-Different-Groups Rule
The most straightforward approach to identifying a chirality center is to systematically examine each carbon atom in the molecule. Ask yourself: Is this carbon atom bonded to four different groups? If yes, it's a chirality center.
Example 1: Consider the molecule 2-chlorobutane.
The second carbon atom is bonded to:
- A methyl group (-CH₃)
- An ethyl group (-CH₂CH₃)
- A chlorine atom (-Cl)
- A hydrogen atom (-H)
Since all four groups are different, this carbon atom is a chirality center.
Example 2: Now consider 2-methylpropane.
The central carbon atom is bonded to:
- Three methyl groups (-CH₃)
- One hydrogen atom (-H)
Because three of the groups are identical (methyl groups), this carbon atom is not a chirality center. The molecule is therefore achiral.
Beyond Carbon: Other Chirality Centers
While carbon is the most common atom forming chirality centers, other atoms can also exhibit chirality. Atoms with four different groups bonded to them, such as silicon, phosphorus, nitrogen, and sulfur, can also serve as chirality centers. However, the conditions for nitrogen and phosphorus are slightly more nuanced due to the possibility of inversion.
Nitrogen as a Chirality Center
Nitrogen atoms can act as chirality centers, but due to their ability to undergo rapid inversion (a process where the nitrogen atom flips its configuration), they are less frequently considered as stereocenters. The inversion process makes the separation and study of nitrogen enantiomers significantly challenging. However, in certain circumstances, particularly in cyclic structures or when the nitrogen atom is part of a rigid system, nitrogen can indeed be a chirality center.
Phosphorus as a Chirality Center
Similar to nitrogen, phosphorus can also be a chirality center. However, the inversion process in phosphorus is considerably slower than in nitrogen. Consequently, phosphorus stereocenters are more easily observed and studied compared to nitrogen stereocenters.
Sulfur as a Chirality Center
Sulfur atoms can also act as chirality centers, though their chirality is often less stable than that of carbon. The inversion barrier for sulfur is relatively low, leading to rapid interconversion between enantiomers in many cases. However, in specific molecular environments, sulfur can act as a chiral center.
Advanced Techniques for Identifying Chirality Centers
While the four-different-groups rule is a great starting point, some molecules may present more complex scenarios. Let's explore some advanced techniques for identifying chirality centers in challenging cases:
Identifying Chirality Centers in Cyclic Molecules
In cyclic molecules, the identification of chirality centers requires careful consideration of the ring structure and its substituents. Each carbon atom within the ring must be evaluated individually, applying the four-different-groups rule. The presence of ring structures can influence the overall chirality of the molecule.
Identifying Chirality Centers with Multiple Chirality Centers
Molecules can possess multiple chirality centers. In these cases, each carbon atom needs to be assessed individually. The number of possible stereoisomers increases exponentially with the number of chirality centers (2<sup>n</sup>, where 'n' is the number of chirality centers). Analyzing molecules with multiple chirality centers often requires advanced stereochemical nomenclature and concepts like diastereomers and meso compounds.
Identifying Pseudoasymmetric Centers
A pseudoasymmetric center is a carbon atom bonded to four different groups, but two of these groups are enantiomers of each other. This special case requires careful examination and understanding of the molecule’s symmetry.
Practical Applications of Identifying Chirality Centers
Understanding and identifying chirality centers is not just an academic exercise; it has profound implications across various scientific fields:
Pharmaceutical Industry
Chirality is crucial in the pharmaceutical industry. Enantiomers of a drug can exhibit significantly different pharmacological activities, toxicities, and metabolic pathways. Identifying chirality centers allows for the development of targeted therapies with improved efficacy and reduced side effects. The infamous thalidomide tragedy highlighted the dramatic consequences of ignoring chirality in drug design.
Food Science and Flavor Chemistry
Chirality also impacts the taste and smell of food molecules. Enantiomers of the same molecule can have distinctly different sensory properties. Understanding the chirality of flavor and fragrance compounds is vital for developing new food products and enhancing existing ones.
Material Science
Chirality plays a role in material science, particularly in the development of chiral materials with unique optical and electronic properties. Identifying chirality centers is vital in designing and synthesizing materials with specific functionalities.
Conclusion: Mastering Chirality Center Identification
Mastering the identification of chirality centers is a cornerstone of organic chemistry. By understanding the fundamental principles outlined in this guide and practicing with diverse molecular structures, you will develop the skills necessary to confidently analyze and predict the stereochemical properties of molecules. Remember to systematically apply the four-different-groups rule, and be prepared to tackle more challenging scenarios, such as cyclic molecules and molecules containing multiple chirality centers. The ability to identify chirality centers is not only crucial for understanding molecular structure but also has far-reaching implications across numerous scientific disciplines. This knowledge forms a solid foundation for further exploration of stereochemistry, a field rich in both theoretical depth and practical applications. Through diligent practice and a keen eye for detail, you can confidently navigate the fascinating world of chiral molecules and their diverse properties.
Latest Posts
Latest Posts
-
How To Subtract Fractions Mixed Numbers
Apr 08, 2025
-
What Are The Properties Of A Gas
Apr 08, 2025
-
Which Functional Group Acts As A Base
Apr 08, 2025
-
Does Staph Aureus Grow On Macconkey Agar
Apr 08, 2025
-
What Phase Of Mitosis Is The Shortest
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Identify The Chirality Center In Each Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.