Identify The Functional Group Found Each Of The Following Molecules
Muz Play
Apr 06, 2025 · 7 min read
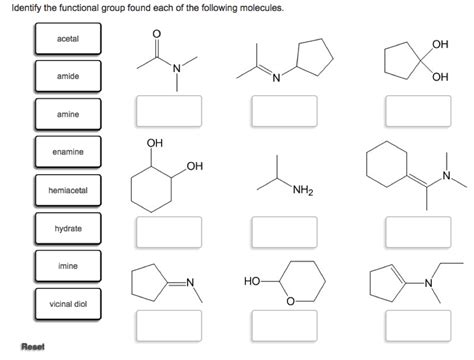
Table of Contents
Identifying Functional Groups in Organic Molecules: A Comprehensive Guide
Organic chemistry, the study of carbon-containing compounds, is built upon the concept of functional groups. These are specific atoms or groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Identifying these functional groups is crucial for predicting the properties and reactivity of organic molecules. This comprehensive guide will walk you through the identification of various functional groups in different organic molecules, providing a solid foundation for understanding organic chemistry.
What are Functional Groups?
Functional groups are specific arrangements of atoms within a molecule that impart characteristic chemical properties. They are the reactive sites of a molecule, dictating how it will behave in chemical reactions. Regardless of the size or complexity of the rest of the molecule, the presence of a specific functional group will largely determine its reactivity. Think of functional groups as the "personality" of a molecule, defining its behavior in the chemical world.
Common Functional Groups and Their Identification
Let's delve into some of the most common functional groups, detailing their structures and how to identify them within a molecule. We'll use structural formulas and examples to illustrate the concepts effectively.
1. Alkanes (C-C, C-H): The Foundation
Alkanes are hydrocarbons containing only single bonds between carbon atoms (C-C) and between carbon and hydrogen atoms (C-H). They are the simplest organic molecules and serve as the basis for understanding more complex structures. They are characterized by their relatively unreactive nature due to the strong and non-polar nature of the C-C and C-H bonds.
Example: Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈)
Identification: Look for molecules containing only carbon and hydrogen atoms connected by single bonds. The absence of any other atom or group indicates an alkane.
2. Alkenes (C=C): Introducing Unsaturation
Alkenes contain at least one carbon-carbon double bond (C=C). This double bond introduces unsaturation, making alkenes significantly more reactive than alkanes. The double bond allows for addition reactions, where atoms or groups add across the double bond.
Example: Ethene (C₂H₄), Propene (C₃H₆)
Identification: The presence of a carbon-carbon double bond is the key identifying feature of alkenes.
3. Alkynes (C≡C): Even More Unsaturation
Alkynes possess at least one carbon-carbon triple bond (C≡C). Like alkenes, alkynes are unsaturated and even more reactive than alkenes due to the presence of the triple bond. They undergo addition reactions similar to alkenes, but often with even greater reactivity.
Example: Ethyne (C₂H₂), Propyne (C₃H₄)
Identification: The presence of a carbon-carbon triple bond is the defining characteristic of alkynes.
4. Alcohols (-OH): The Hydroxyl Group
Alcohols contain a hydroxyl group (-OH) attached to a carbon atom. The hydroxyl group is polar, making alcohols more soluble in water than alkanes. The -OH group also allows for various reactions, such as oxidation and esterification.
Example: Methanol (CH₃OH), Ethanol (C₂H₅OH), Propanol (C₃H₇OH)
Identification: Look for an -OH group directly bonded to a carbon atom.
5. Ethers (-O-): Linking Carbon Atoms
Ethers contain an oxygen atom (O) bonded to two carbon atoms (-O-). They are relatively unreactive compared to alcohols, but their polar nature contributes to some solubility in water.
Example: Dimethyl ether (CH₃OCH₃), Diethyl ether (C₂H₅OC₂H₅)
Identification: Identify the presence of an oxygen atom bonded to two carbon atoms. The oxygen atom is not bonded to a hydrogen atom.
6. Aldehydes (-CHO): Terminal Carbonyl Group
Aldehydes have a carbonyl group (C=O) at the end of a carbon chain. The carbonyl carbon is bonded to at least one hydrogen atom. Aldehydes are readily oxidized, a key characteristic used in many chemical tests.
Example: Formaldehyde (HCHO), Acetaldehyde (CH₃CHO)
Identification: Look for a carbonyl group (C=O) at the end of a carbon chain. The carbonyl carbon must be bonded to at least one hydrogen atom.
7. Ketones (C=O): Internal Carbonyl Group
Ketones also contain a carbonyl group (C=O), but the carbonyl carbon is bonded to two other carbon atoms. This positions the carbonyl group within the carbon chain, unlike aldehydes. Ketones are less reactive than aldehydes.
Example: Acetone (CH₃COCH₃), Butanone (CH₃COC₂H₅)
Identification: Identify a carbonyl group (C=O) where the carbonyl carbon is bonded to two other carbon atoms.
8. Carboxylic Acids (-COOH): Acidic Functionality
Carboxylic acids possess a carboxyl group (-COOH), which is a combination of a carbonyl group (C=O) and a hydroxyl group (-OH) on the same carbon atom. Carboxylic acids are acidic and readily lose a proton (H⁺) in solution.
Example: Formic acid (HCOOH), Acetic acid (CH₃COOH)
Identification: Look for the -COOH group. This is a combination of a carbonyl group and a hydroxyl group on the same carbon.
9. Esters (-COO-): Fruity Scents
Esters contain a carbonyl group (C=O) bonded to an oxygen atom, which is further bonded to another carbon atom (-COO-). Esters are often associated with pleasant fruity odors and are commonly found in fragrances and flavors.
Example: Methyl acetate (CH₃COOCH₃), Ethyl acetate (CH₃COOC₂H₅)
Identification: Look for the -COO- group, which consists of a carbonyl group bonded to an oxygen atom bonded to another carbon.
10. Amines (-NH₂, -NHR, -NR₂): Nitrogen-Containing Groups
Amines contain a nitrogen atom bonded to one, two, or three carbon atoms (-NH₂, -NHR, -NR₂). The number of carbon atoms bonded to the nitrogen determines the classification of the amine (primary, secondary, or tertiary). Amines are basic and can accept a proton (H⁺) in solution.
Example: Methylamine (CH₃NH₂), Dimethylamine (CH₃)₂NH, Trimethylamine (CH₃)₃N
Identification: Look for a nitrogen atom bonded to one or more carbon atoms and potentially hydrogen atoms.
11. Amides (-CONH₂): Peptide Bonds
Amides contain a carbonyl group (C=O) bonded to a nitrogen atom, which is further bonded to one or two carbon atoms or hydrogen atoms (-CONH₂). Amide bonds are crucial in the structure of proteins and peptides.
Example: Formamide (HCONH₂), Acetamide (CH₃CONH₂)
Identification: Look for the -CONH₂ group, which contains a carbonyl group bonded to a nitrogen atom.
12. Nitriles (-CN): Triple Bond to Nitrogen
Nitriles possess a cyano group (-CN), which consists of a carbon atom triple-bonded to a nitrogen atom. The triple bond makes nitriles relatively reactive.
Example: Acetonitrile (CH₃CN)
Identification: Identify the presence of the -CN group, indicating a carbon-nitrogen triple bond.
13. Halogenated Compounds (-F, -Cl, -Br, -I): Halogens Attached
These compounds contain halogen atoms (fluorine, chlorine, bromine, or iodine) attached to carbon atoms. The presence of halogens significantly alters the properties of the molecule, often increasing its reactivity.
Example: Chloromethane (CH₃Cl), Bromomethane (CH₃Br)
Identification: Look for the presence of fluorine (F), chlorine (Cl), bromine (Br), or iodine (I) atoms bonded to carbon atoms.
14. Nitro Compounds (-NO₂): Explosive Potential
Nitro compounds contain a nitro group (-NO₂), which is a nitrogen atom double-bonded to two oxygen atoms. Many nitro compounds are explosive and are used in various applications, including explosives and propellants.
Example: Nitromethane (CH₃NO₂)
Identification: Look for the -NO₂ group, consisting of a nitrogen atom double-bonded to two oxygen atoms.
15. Sulfides (-S-): Sulfur Analogs of Ethers
Sulfides contain a sulfur atom (S) bonded to two carbon atoms (-S-). They are sulfur analogs of ethers and have somewhat similar properties.
Example: Dimethyl sulfide (CH₃SCH₃)
Identification: Identify the presence of a sulfur atom bonded to two carbon atoms.
16. Thiols (-SH): Sulfur Analogs of Alcohols
Thiols contain a sulfhydryl group (-SH), which is a sulfur analog of the hydroxyl group in alcohols. Thiols are known for their characteristic unpleasant odors.
Example: Methanethiol (CH₃SH)
Identification: Look for a sulfur atom bonded to a hydrogen atom (-SH).
Practical Tips for Identifying Functional Groups
-
Systematic Approach: Start by identifying the carbon skeleton and then look for specific atoms or groups attached to it.
-
Prioritize: Some functional groups take precedence over others (e.g., carboxylic acids over alcohols).
-
Look for Characteristic Patterns: Practice recognizing patterns within the structures.
-
Use Online Resources: Various online tools and databases can aid in the identification of functional groups in complex molecules.
-
Practice, Practice, Practice: The key to mastering functional group identification is through consistent practice with a variety of molecules.
Conclusion: Mastering Functional Groups
The ability to identify functional groups is fundamental to understanding organic chemistry. This comprehensive guide has equipped you with the knowledge to recognize and classify a wide range of common functional groups. By applying the strategies outlined here, you can confidently analyze organic molecules and predict their chemical behavior. Remember that consistent practice is crucial to build proficiency in this essential aspect of organic chemistry. As you gain experience, you will become adept at recognizing even complex molecules' functional groups at a glance. Good luck!
Latest Posts
Latest Posts
-
Given The Kinetics Data For Each Enzyme
Apr 09, 2025
-
What Are Turning Points In A Graph
Apr 09, 2025
-
What Percent Of Alcohol Is Absorbed Through The Small Intestine
Apr 09, 2025
-
What State Of Matter Has No Definite Shape Or Volume
Apr 09, 2025
-
Polynomials That Cannot Be Factored Are Called
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Identify The Functional Group Found Each Of The Following Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.