Identify The Resulting Resonance Structure Indicated By The Curved Arrows
Muz Play
Apr 07, 2025 · 6 min read
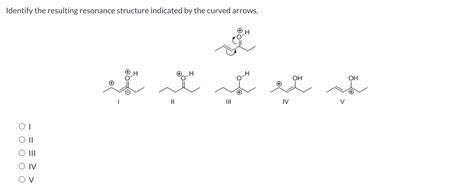
Table of Contents
Identifying Resulting Resonance Structures Indicated by Curved Arrows
Resonance structures are crucial in understanding the behavior of molecules, particularly those containing conjugated pi systems. They represent the delocalization of electrons within a molecule, leading to a more stable overall structure than any single Lewis structure can depict. Understanding how to interpret curved arrows and predict resulting resonance structures is fundamental to organic chemistry. This comprehensive guide will walk you through the process, covering various examples and nuances.
Understanding Curved Arrows
Curved arrows are a shorthand notation used in organic chemistry to illustrate the movement of electrons within a molecule. They are essential for depicting resonance structures, reaction mechanisms, and electron flow in general.
Types of Electron Movement
- Movement of a single electron: Represented by a single-headed arrow (•→). This is commonly seen in radical reactions.
- Movement of a pair of electrons: Represented by a double-headed arrow (→). This is the most common type used for depicting resonance and the movement of electron pairs in reactions.
Rules for Using Curved Arrows
- Arrows originate from an electron-rich area: This is typically a lone pair of electrons, a pi bond, or a negative charge.
- Arrows point to an electron-deficient area: This is often a positive charge, a partially positive atom, or an atom with an incomplete octet.
- Arrows show the movement of electrons, not atoms: The atoms remain in the same positions; only the electrons move.
- Maintain formal charges: When moving electrons, remember to adjust the formal charges on the atoms involved accordingly.
Identifying Resonance Structures: A Step-by-Step Guide
Let's break down the process of identifying resulting resonance structures indicated by curved arrows with several examples.
Example 1: Simple Resonance in a Conjugated System
Consider the following molecule with a curved arrow indicating the movement of a pi electron pair:
O
||
CH₂=C−C−CH₃
|
H
with a curved arrow from the double bond to the carbonyl oxygen.
Step 1: Identify the electron source: The pi bond between the carbon atoms is the electron-rich area.
Step 2: Identify the electron sink: The carbonyl oxygen has a partially positive charge due to its electronegativity and the polar C=O bond; it’s the electron-deficient area.
Step 3: Move the electrons: Follow the curved arrow. The pi bond breaks, and the electrons move to form a new pi bond between the carbon and the oxygen. The original carbon atom now has a positive charge.
Resulting Resonance Structure:
O⁻
|
CH₂−C=C−CH₃
|
H⁺
Notice how the formal charges have been updated to reflect the movement of electrons. This resonance structure shows the delocalization of the electrons, contributing to the overall stability of the molecule.
Example 2: Resonance with Lone Pairs
Let's analyze a molecule with a lone pair participating in resonance:
O
||
C−N=O
|
H
with a curved arrow moving a lone pair from the oxygen to form a double bond with the adjacent carbon.
Step 1: Electron Source: The lone pair of electrons on the oxygen atom.
Step 2: Electron Sink: The C=N double bond provides the electron-deficient area.
Step 3: Electron Movement: The lone pair forms a new pi bond between the oxygen and carbon, while the existing pi bond in the C=N shifts to become a lone pair on the nitrogen.
Resulting Resonance Structure:
O⁻
||
C−N⁺−O
|
H
This illustrates resonance involving lone pair participation, creating different charge distributions across the molecule.
Example 3: More Complex Resonance Systems
Consider a conjugated system with multiple pi bonds:
O
||
CH₂=CH−CH=CH−C−CH₃
|
H
with a curved arrow moving a pair of pi electrons from the leftmost double bond towards the adjacent single bond.
This example demonstrates a more complex situation with a longer conjugated system. The movement of electrons needs to be carefully tracked to avoid mistakes. The first step results in a shift of electron density across the chain. Further resonance structures can be drawn, continuing to delocalize the electrons along the chain. Generating all possible resonance contributors is a crucial skill in understanding the reactivity and stability of such molecules.
Resulting Resonance Structure (after one arrow's movement):
O
||
CH₂−CH=CH−CH=C−CH₃
|
H
This process can be repeated with another curved arrow to further show the delocalization of electron density along the pi system. Remember that resonance structures always contribute to the actual structure, which is a hybrid of all the contributing forms.
Example 4: Resonance in Aromatic Systems
Aromatic compounds, such as benzene, exhibit extensive resonance stabilization. Understanding how curved arrows indicate electron movement in these systems is crucial.
Benzene can be represented by two resonance structures:
H H
| |
H−C=C−C=C−C=C−H ↔ H−C−C=C−C=C−C−H
| |
H H
Each structure shows a distinct placement of double and single bonds. The true benzene structure is a hybrid of these two, with the electrons delocalized across the entire ring. Curved arrows representing the movement of electron pairs can illustrate the interconversion between these resonance structures.
Advanced Concepts in Resonance
Major and Minor Resonance Contributors
Not all resonance structures contribute equally to the overall structure. Factors determining the relative importance include:
- Octet Rule: Structures where atoms have complete octets are generally more important.
- Formal Charges: Structures with minimal formal charges are favored.
- Electronegativity: Negative charges are more stable on electronegative atoms, and positive charges are more stable on electropositive atoms.
Structures that satisfy these criteria more effectively are considered major contributors.
Resonance Hybrid
The actual structure of a molecule with resonance is not any single resonance structure, but rather a weighted average of all contributors. This is known as the resonance hybrid. The hybrid is typically more stable than any individual contributing structure.
Practical Applications of Resonance Theory
Understanding resonance is crucial for:
- Predicting molecular stability: Resonance stabilization plays a significant role in determining the reactivity and stability of various molecules.
- Understanding reaction mechanisms: Resonance structures help to visualize the movement of electrons during chemical reactions.
- Interpreting spectroscopic data: Resonance influences molecular properties that are measurable by spectroscopic techniques.
- Designing and synthesizing new molecules: Understanding resonance is important for designing molecules with desired properties.
Conclusion
Identifying resonance structures using curved arrows is a fundamental skill in organic chemistry. By systematically following the rules of electron movement and considering the relative stability of different contributors, you can effectively predict and interpret the resonance structures of a wide range of molecules. This knowledge is essential for understanding the behavior and reactivity of molecules, and it underpins much of advanced organic chemistry. Mastering the interpretation of curved arrows and the concepts discussed here will significantly improve your understanding of chemical bonding and molecular properties. Remember that practice is key – work through numerous examples to solidify your understanding. The more comfortable you become with visualizing electron movement, the easier it will be to predict resonance structures and understand their implications.
Latest Posts
Latest Posts
-
Three Steps Of The Perception Process
Apr 09, 2025
-
How To Tell If Something Is Axial Or Equatorial
Apr 09, 2025
-
Is Pool Water A Homogeneous Mixture
Apr 09, 2025
-
Fungi Can Reproduce Both Sexually And Asexually
Apr 09, 2025
-
What Is The Driving Force Of Evolution
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Identify The Resulting Resonance Structure Indicated By The Curved Arrows . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.