If An Atom Gains An Electron It Becomes
Muz Play
Apr 06, 2025 · 6 min read
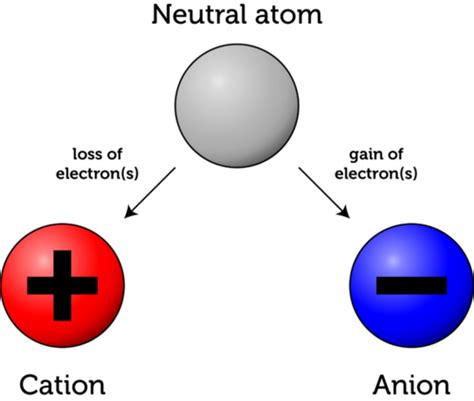
Table of Contents
If an Atom Gains an Electron, It Becomes... an Ion! Understanding Ionization and its Implications
Have you ever wondered what happens at the subatomic level when an atom interacts with its environment? One fundamental process is the gain or loss of electrons. This seemingly simple event has profound consequences, transforming the atom's properties and influencing its behavior in countless chemical and physical processes. Let's delve into the fascinating world of ionization, exploring what happens when an atom gains an electron, and the implications of this transformation.
Understanding Atomic Structure: The Foundation of Ionization
Before exploring the consequences of electron gain, it's crucial to understand the basic structure of an atom. At its core is the nucleus, containing positively charged protons and neutral neutrons. Surrounding the nucleus is a cloud of negatively charged electrons, arranged in specific energy levels or shells. The number of protons in an atom's nucleus defines its atomic number and determines the element. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon. Atoms are typically electrically neutral, meaning the number of protons equals the number of electrons. This balance of positive and negative charges maintains overall neutrality.
The Role of Valence Electrons
The electrons in the outermost shell, known as valence electrons, are particularly important in chemical reactions and ionization. These electrons are loosely bound to the atom and readily participate in interactions with other atoms. The number of valence electrons determines an atom's reactivity and its tendency to gain or lose electrons to achieve a stable electron configuration, often referred to as the "octet rule" (eight valence electrons).
What Happens When an Atom Gains an Electron?
When a neutral atom gains an electron, it acquires an extra negative charge. This process is called reduction, and the resulting atom is no longer neutral. It now carries a net negative charge and is called an anion. The anion's overall charge is denoted by a superscript minus sign followed by the number of extra electrons gained. For example, if a chlorine atom (Cl) gains one electron, it becomes a chloride ion (Cl⁻). If it gains two electrons, it would be denoted as Cl²⁻.
The Mechanics of Electron Gain
Several mechanisms can lead to an atom gaining an electron. One common mechanism involves the transfer of electrons from one atom to another. This often occurs in reactions between atoms with significantly different electronegativities. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity tend to attract electrons more strongly, increasing their likelihood of gaining electrons in a reaction.
Another mechanism is through the absorption of energy, such as light or heat. If an atom absorbs sufficient energy, it can excite an electron to a higher energy level, leaving a vacancy in its original shell. This vacancy can then be filled by an incoming free electron, leading to the formation of an anion.
The Properties of Anions: A Transformation of the Atom
The gain of an electron fundamentally alters the atom's properties. Several key changes occur:
-
Charge: The most obvious change is the acquisition of a net negative charge. This negative charge dramatically alters the atom's interactions with other atoms and molecules.
-
Size: Anions are generally larger than their neutral counterparts. The added electron increases the electron-electron repulsion, causing the electron cloud to expand. This increase in size affects the anion's reactivity and its interactions in solutions.
-
Electronegativity: The electronegativity of an anion is lower than that of its corresponding neutral atom. Since it already possesses an extra electron, its attraction for additional electrons is reduced.
-
Reactivity: The reactivity of an anion can differ significantly from its neutral atom. Anions often exhibit different bonding preferences and participate in different chemical reactions compared to their neutral forms. For instance, the chloride ion (Cl⁻) forms ionic bonds with many metal cations, a property not shared by the neutral chlorine atom.
Examples of Anion Formation: Real-World Applications
The formation of anions is ubiquitous in chemistry and plays a crucial role in numerous processes. Here are a few examples illustrating the significance of this phenomenon:
-
Salt Formation: The formation of common table salt (NaCl) involves the transfer of an electron from a sodium atom (Na) to a chlorine atom (Cl). Sodium loses an electron to become a sodium cation (Na⁺), while chlorine gains an electron to become a chloride anion (Cl⁻). The electrostatic attraction between these oppositely charged ions forms the ionic compound sodium chloride.
-
Electrolyte Solutions: Anions are essential components of electrolyte solutions, which are crucial for various biological processes and industrial applications. These solutions conduct electricity because the ions are free to move and carry charge. Examples include the sodium and chloride ions in bodily fluids and the ions in batteries.
-
Acid-Base Reactions: Many acid-base reactions involve the transfer of protons (H⁺) and the formation of anions. When an acid donates a proton, the remaining part of the molecule becomes an anion. For example, when hydrochloric acid (HCl) dissociates in water, it forms a hydronium ion (H₃O⁺) and a chloride ion (Cl⁻).
-
Oxidation-Reduction Reactions (Redox Reactions): Reduction (gain of electrons) is one half of the redox reaction. These reactions are fundamental to many biological and industrial processes, including respiration, photosynthesis, and corrosion. Understanding the formation of anions is crucial in comprehending redox reactions and their implications.
Beyond Anions: Cations and Isoelectronic Species
It's important to note that the converse of anion formation—the loss of an electron—also occurs. When an atom loses an electron, it becomes a cation, carrying a net positive charge. The formation of both cations and anions is vital in forming ionic compounds.
Furthermore, atoms or ions with the same number of electrons are called isoelectronic species. For instance, the fluoride ion (F⁻), the neon atom (Ne), and the sodium ion (Na⁺) all have 10 electrons, making them isoelectronic. Isoelectronic species often exhibit similar chemical properties due to their identical electron configurations.
Conclusion: The Importance of Ionization in Chemistry and Beyond
The process of an atom gaining an electron, leading to the formation of an anion, is a fundamental concept in chemistry with widespread implications. This seemingly simple event profoundly alters the atom's properties, affecting its size, charge, reactivity, and interactions with other atoms and molecules. Understanding ionization is crucial for comprehending a vast array of chemical reactions, biological processes, and industrial applications. From the formation of salts to the functioning of electrolyte solutions and the intricacies of redox reactions, the impact of electron gain is far-reaching and essential to our understanding of the material world. The study of ionization continues to be a vital area of research, furthering our understanding of the fundamental interactions that shape the universe.
Latest Posts
Latest Posts
-
Collection Of Nerve Cell Bodies In The Peripheral Nervous System
Apr 08, 2025
-
Which Of The Following Is True Of Spending In Politics
Apr 08, 2025
-
Find The Tension In Rope A
Apr 08, 2025
-
Stimulation Of The Heat Loss Center Causes
Apr 08, 2025
-
Light Dependent And Light Independent Differences
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about If An Atom Gains An Electron It Becomes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.