If Keq Is Greater Than 1
Muz Play
Mar 31, 2025 · 6 min read
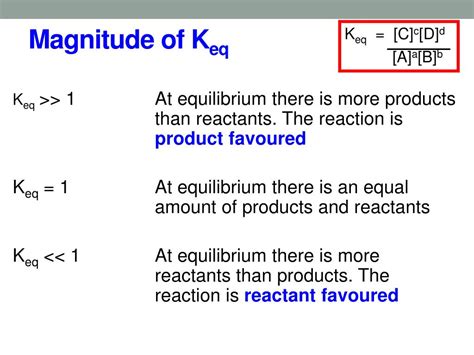
Table of Contents
If Keq is Greater Than 1: Understanding Equilibrium Constants and Their Significance
The equilibrium constant, Keq, is a fundamental concept in chemistry that quantifies the relative amounts of reactants and products present at equilibrium for a reversible reaction. A reversible reaction is one that can proceed in both the forward and reverse directions. Understanding the value of Keq, specifically when it's greater than 1, is crucial for predicting the direction and extent of a reaction. This article will delve into the meaning of Keq > 1, its implications, factors influencing it, and its applications in various chemical processes.
What Does Keq Represent?
Keq is a dimensionless quantity that represents the ratio of the concentrations of products to reactants at equilibrium, each raised to the power of its stoichiometric coefficient in the balanced chemical equation. For a general reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
Keq = ([C]^c * [D]^d) / ([A]^a * [B]^b)
where [A], [B], [C], and [D] represent the equilibrium concentrations of reactants A, B and products C, D respectively. The exponents a, b, c, and d are the stoichiometric coefficients from the balanced equation.
The Significance of Keq > 1
When Keq > 1, it indicates that the equilibrium lies to the right, favoring the formation of products. In other words, at equilibrium, the concentration of products is significantly higher than the concentration of reactants. This means that the forward reaction (reactants forming products) is more favorable than the reverse reaction (products forming reactants). The larger the value of Keq, the more the equilibrium favors the products. A very large Keq indicates that the reaction essentially goes to completion, with almost all reactants being converted into products.
Factors Affecting Keq
Several factors can influence the value of Keq:
1. Temperature:
Temperature is a crucial factor affecting the equilibrium constant. The effect of temperature on Keq depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
-
Exothermic Reactions: For exothermic reactions, increasing the temperature shifts the equilibrium to the left (favoring reactants), thus decreasing Keq. Decreasing the temperature shifts the equilibrium to the right (favoring products), increasing Keq.
-
Endothermic Reactions: For endothermic reactions, increasing the temperature shifts the equilibrium to the right (favoring products), increasing Keq. Decreasing the temperature shifts the equilibrium to the left (favoring reactants), decreasing Keq.
This relationship is governed by the van 't Hoff equation.
2. Pressure (for gaseous reactions):
For reactions involving gases, changes in pressure can affect Keq. Increasing the pressure favors the side with fewer gas molecules, while decreasing the pressure favors the side with more gas molecules. This is because the system will adjust to relieve the stress imposed by the pressure change. However, it's important to note that pressure changes do not affect Keq directly; they shift the equilibrium position. Only temperature changes directly alter Keq.
3. Concentration:
Changing the concentration of reactants or products will shift the equilibrium to counteract the change. Adding more reactants will shift the equilibrium to the right (favoring products), while adding more products will shift the equilibrium to the left (favoring reactants). However, this again only shifts the equilibrium; Keq remains constant at a constant temperature.
Applications of Keq > 1
The knowledge that Keq > 1 has numerous practical applications across various chemical domains:
1. Industrial Processes:
Many industrial chemical processes are designed to maximize product formation. Reactions with Keq > 1 are ideal for such applications because they naturally favor product formation. Examples include the Haber-Bosch process for ammonia synthesis and the production of sulfuric acid. In these cases, reaction conditions (temperature, pressure, concentration) are carefully optimized to maximize Keq and achieve high yields.
2. Pharmaceutical Drug Development:
In pharmaceutical drug development, Keq plays a crucial role in understanding the binding affinity of drugs to their target receptors. A high Keq value indicates a strong binding affinity, which is essential for effective drug action. Researchers use Keq values to evaluate and compare different drug candidates.
3. Environmental Chemistry:
Keq values are important for understanding various environmental processes, such as the solubility of pollutants in water and the equilibrium between different forms of chemical species in the atmosphere or soil. Knowing Keq helps predict the fate and transport of pollutants and design effective remediation strategies.
4. Analytical Chemistry:
Keq is used extensively in analytical chemistry for developing quantitative analytical methods. For instance, in titrations, the equilibrium constant of the reaction between the analyte and titrant determines the sharpness of the endpoint. A larger Keq implies a sharper endpoint, improving the accuracy of the analysis.
Understanding the Limitations of Keq
While Keq is a powerful tool for predicting reaction behavior, it has limitations:
-
Keq only provides information about the relative amounts of reactants and products at equilibrium, not the rate of the reaction. A reaction with a large Keq can be very slow, and vice versa. Reaction kinetics is needed to understand the rate.
-
Keq is only valid for a specific temperature. Changes in temperature alter Keq.
-
Keq is only applicable to systems at equilibrium. It cannot be used to predict the composition of a reaction mixture that is not at equilibrium.
Connecting Keq to Gibbs Free Energy
The standard Gibbs free energy change (ΔG°) is directly related to the equilibrium constant through the following equation:
ΔG° = -RTlnKeq
where:
- R is the ideal gas constant
- T is the temperature in Kelvin
This equation highlights the thermodynamic basis of Keq. A negative ΔG° corresponds to a Keq > 1, indicating a spontaneous reaction (under standard conditions). Conversely, a positive ΔG° corresponds to a Keq < 1, indicating a non-spontaneous reaction (under standard conditions). A ΔG° of zero corresponds to a Keq of 1, indicating that the reaction is at equilibrium.
Beyond Simple Equilibrium: Complex Systems
The concept of Keq extends beyond simple reactions to more complex systems involving multiple equilibria. In such cases, multiple equilibrium constants need to be considered to describe the overall behavior of the system. For instance, in the study of acid-base equilibria, multiple protonation steps are often involved, each characterized by its own Keq.
Conclusion:
A Keq greater than 1 signifies a reaction that favors product formation at equilibrium. This understanding is crucial in various fields, from industrial processes to pharmaceutical drug development and environmental science. While Keq provides valuable insights into reaction behavior, it's essential to consider its limitations and to integrate it with other chemical concepts, such as reaction kinetics and thermodynamics, for a comprehensive understanding of chemical systems. The relationship between Keq and Gibbs Free Energy further strengthens this understanding, providing a thermodynamic perspective on the spontaneity of chemical reactions. The importance of Keq extends to complex systems, requiring a deeper understanding of multiple equilibria.
Latest Posts
Latest Posts
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
-
In A Solution It Is Dissolving Medium
Apr 02, 2025
-
How To Find Moles Of Naoh Used In Titration
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about If Keq Is Greater Than 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
