Ions With Positive Charge Are Called
Muz Play
Mar 17, 2025 · 5 min read
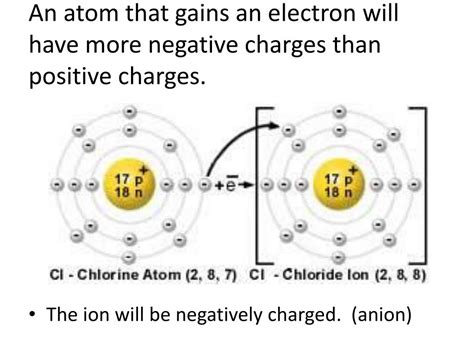
Table of Contents
Ions with a Positive Charge are Called Cations: A Deep Dive into the World of Ionic Chemistry
Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net electrical charge. This fundamental concept underpins countless chemical reactions and processes, from the functioning of biological systems to the creation of advanced materials. Understanding ions is crucial for grasping a wide range of scientific principles. This article delves into the world of ions, focusing specifically on those with a positive charge – cations.
What are Ions? The Basics of Ionic Charge
Before we explore cations in detail, let's establish a firm foundation by defining what an ion is. An atom in its neutral state contains an equal number of protons (positively charged particles in the nucleus) and electrons (negatively charged particles orbiting the nucleus). When an atom gains or loses electrons, this balance is disrupted, creating an ion.
-
Anions: When an atom gains one or more electrons, it acquires a negative charge and is called an anion. The extra electron(s) outweigh the positive charge of the protons. Common examples include chloride ions (Cl⁻) and oxide ions (O²⁻).
-
Cations: Conversely, when an atom loses one or more electrons, it loses negative charge, leaving behind a net positive charge. This positively charged ion is called a cation. The positive charge of the protons now outweighs the negative charge of the remaining electrons. Examples include sodium ions (Na⁺) and calcium ions (Ca²⁺).
Cations: A Closer Look at Positively Charged Ions
Cations are formed when atoms, typically metals, lose electrons to achieve a more stable electron configuration. This often involves attaining a full outer electron shell (octet rule), mirroring the stable electron configuration of noble gases. The tendency to lose electrons and form cations is linked to an element's electronegativity; elements with low electronegativity readily lose electrons.
Formation of Cations: The Mechanism
The formation of a cation involves the removal of one or more electrons from the outermost electron shell (valence shell) of an atom. This process requires energy, as the electrons are attracted to the positively charged nucleus. However, the resulting cation often has a lower overall energy state due to its stable electron configuration. The energy required to remove an electron is called the ionization energy.
For example, consider sodium (Na). Its neutral atom has 11 electrons. By losing one electron from its valence shell, it forms a sodium cation (Na⁺) with 10 electrons, achieving a stable electron configuration similar to neon. This process is depicted in the following simplified equation:
Na → Na⁺ + e⁻
Naming Cations: Simple and Complex Ions
Naming cations is generally straightforward. For monatomic cations (cations formed from a single atom), the name of the element is followed by the word "ion" and the charge is indicated using Roman numerals in parentheses if the element has multiple oxidation states (can form ions with different charges). For example:
- Sodium ion (Na⁺)
- Calcium ion (Ca²⁺)
- Iron(II) ion (Fe²⁺) - Note the use of Roman numerals to distinguish from Iron(III) ion (Fe³⁺)
- Aluminum ion (Al³⁺)
Polyatomic cations (cations composed of multiple atoms) have specific names that you need to learn. A prominent example is the ammonium ion (NH₄⁺).
Properties and Behavior of Cations
The properties and behavior of cations are significantly influenced by their charge and size.
Charge and Size: Impact on Properties
The magnitude of the positive charge influences the cation's interaction with other ions and molecules. Higher charged cations exert stronger electrostatic forces, affecting their solubility, reactivity, and coordination chemistry. Similarly, the size of the cation plays a role; smaller cations generally have a higher charge density, leading to stronger interactions.
Role in Chemical Reactions
Cations are essential participants in a vast range of chemical reactions. Their positive charge facilitates interactions with anions, forming ionic compounds. These compounds are held together by strong electrostatic attractions between oppositely charged ions. The strength of this attraction determines many properties of the ionic compound, including melting point and solubility.
Cations in Biological Systems
Cations are crucial for the proper functioning of biological systems. For example, sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions play vital roles in nerve impulse transmission, muscle contraction, and enzyme activity. These ions are transported across cell membranes via various mechanisms, maintaining the delicate balance necessary for cellular function. Disruptions in cation balance can lead to various health problems.
Examples of Common Cations and Their Applications
Let's examine some examples of common cations and their applications in various fields.
Sodium Ion (Na⁺):
- Ubiquitous in nature: Found in table salt (NaCl) and many other minerals.
- Essential nutrient: Plays crucial roles in fluid balance and nerve impulse transmission.
- Industrial applications: Used in the production of various chemicals, including sodium hydroxide (NaOH) and sodium carbonate (Na₂CO₃).
Potassium Ion (K⁺):
- Essential nutrient: Crucial for muscle function, nerve transmission, and maintaining fluid balance.
- Fertilizer component: Potassium salts are important components of fertilizers, providing potassium to plants.
- Electrolyte in batteries: Used in various battery technologies.
Calcium Ion (Ca²⁺):
- Structural component of bones and teeth: Essential for bone strength and density.
- Muscle contraction: Plays a critical role in muscle contraction and relaxation.
- Blood clotting: Essential for blood clotting.
- Cement and concrete: Calcium compounds are major components of cement and concrete.
Magnesium Ion (Mg²⁺):
- Essential nutrient: Important for enzyme activity, muscle and nerve function, and protein synthesis.
- Plant nutrient: Essential for chlorophyll formation and plant growth.
- Alloying agent: Used in various metal alloys.
Iron Ions (Fe²⁺ and Fe³⁺):
- Oxygen transport: Hemoglobin, the protein responsible for oxygen transport in blood, contains iron ions.
- Enzyme cofactors: Iron ions act as cofactors for various enzymes.
- Steel production: Iron is a key component of steel and other iron alloys.
Ammonium Ion (NH₄⁺):
- Fertilizer component: Ammonium salts are important nitrogen sources in fertilizers.
- Acid-base chemistry: Acts as a weak acid in solution.
Conclusion: The Importance of Cations in Chemistry and Beyond
Cations are fundamental building blocks of matter, playing critical roles in numerous chemical reactions and biological processes. Their positive charge governs their interactions with other ions and molecules, influencing properties and behavior. From the formation of ionic compounds to their essential roles in biological systems, understanding cations is crucial for advancing knowledge in chemistry, biology, materials science, and numerous other scientific fields. The diversity of cations and their widespread applications highlight their enduring importance in our world. Further exploration into the specific properties and applications of individual cations will continue to unlock new insights and innovations.
Latest Posts
Latest Posts
-
Three Parts Of An Rna Nucleotide
Mar 18, 2025
-
Electric Field Of A Line Charge
Mar 18, 2025
-
What Are The 3 Parts Of An Rna Nucleotide
Mar 18, 2025
-
When Do You Use Parentheses In Writing A Chemical Formula
Mar 18, 2025
-
Which Process Takes Place In Chloroplasts
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Ions With Positive Charge Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
