Is A Negative Delta H Exothermic
Muz Play
Mar 15, 2025 · 5 min read
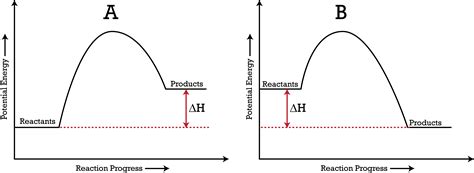
Table of Contents
Is a Negative Delta H Exothermic? Understanding Enthalpy Changes
The question, "Is a negative delta H exothermic?" is a fundamental concept in chemistry and thermodynamics. The short answer is a resounding yes. A negative ΔH (delta H) definitively indicates an exothermic reaction or process. However, understanding why this is true requires delving into the definitions of enthalpy, exothermic reactions, and the crucial relationship between them. This comprehensive guide will explore these concepts, providing a detailed explanation supported by examples and practical applications.
Understanding Enthalpy (H)
Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's a crucial concept for understanding energy changes in chemical reactions and physical processes. We can't directly measure the absolute enthalpy of a substance, but we can easily measure changes in enthalpy (ΔH). These changes are what truly matter when analyzing reactions. ΔH represents the difference in enthalpy between the products and reactants of a process.
The Enthalpy Change (ΔH)
The enthalpy change (ΔH) is calculated as:
ΔH = H<sub>products</sub> - H<sub>reactants</sub>
This equation means that the enthalpy change is the difference between the total enthalpy of the products and the total enthalpy of the reactants. The value of ΔH provides critical information about the energy transfer during a process:
- Negative ΔH: Indicates that the enthalpy of the products is lower than the enthalpy of the reactants. This means that energy is released during the process.
- Positive ΔH: Indicates that the enthalpy of the products is higher than the enthalpy of the reactants. This means that energy is absorbed during the process.
Exothermic and Endothermic Reactions: A Clear Distinction
Chemical reactions are broadly categorized as either exothermic or endothermic based on their enthalpy changes:
Exothermic Reactions: Releasing Energy
An exothermic reaction is one that releases energy to its surroundings. This release of energy is typically in the form of heat, resulting in an increase in the temperature of the surroundings. The system loses energy, and the surroundings gain energy. Think of it like a bonfire: the wood burns (the reaction), releasing heat and light into the surrounding environment. This is an exothermic process with a negative ΔH.
Key characteristics of exothermic reactions:
- Negative ΔH: This is the defining characteristic.
- Heat released: Energy is transferred from the system to the surroundings.
- Temperature increase: The surroundings get hotter.
- Examples: Combustion reactions (like burning fuel), neutralization reactions (acid-base reactions), many oxidation reactions.
Endothermic Reactions: Absorbing Energy
An endothermic reaction is one that absorbs energy from its surroundings. This absorption of energy causes a decrease in the temperature of the surroundings. The system gains energy, and the surroundings lose energy. An example is photosynthesis: plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This process is endothermic and has a positive ΔH.
Key characteristics of endothermic reactions:
- Positive ΔH: This is the defining characteristic.
- Heat absorbed: Energy is transferred from the surroundings to the system.
- Temperature decrease: The surroundings get colder.
- Examples: Photosynthesis, melting ice, dissolving ammonium nitrate in water.
The Inseparable Link: Negative ΔH and Exothermic Processes
The direct and unequivocal link between a negative ΔH and an exothermic process is undeniable. When a reaction or process has a negative enthalpy change, it signifies that the system has lost energy to its surroundings. This energy loss manifests as heat released, thus fulfilling the definition of an exothermic reaction.
Illustrative Examples:
Let's consider two examples to further solidify this understanding:
1. Combustion of Methane:
The combustion of methane (CH₄) is a highly exothermic reaction:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l) ΔH = -890 kJ/mol
The negative ΔH of -890 kJ/mol clearly shows that this reaction releases a significant amount of energy as heat. The surroundings (the environment) experience a considerable temperature increase.
2. Neutralization Reaction:
The reaction between a strong acid (like hydrochloric acid, HCl) and a strong base (like sodium hydroxide, NaOH) is also exothermic:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) ΔH < 0 (negative)
Again, the negative ΔH indicates that heat is released during the neutralization reaction. You can even feel the heat generated if you perform this reaction carefully in a beaker.
Beyond Simple Reactions: Understanding Enthalpy Changes in Complex Systems
While the examples above illustrate simple reactions, the principle of a negative ΔH indicating an exothermic process holds true for far more complex systems and processes. For instance:
- Phase transitions: The freezing of water (liquid to solid) is an exothermic process because the system releases energy as heat during the phase change. The ΔH for this process is negative.
- Nuclear reactions: Nuclear fission, the splitting of an atom's nucleus, is a highly exothermic process, releasing enormous amounts of energy. The ΔH is vastly negative in these cases.
- Biological processes: Many metabolic processes in living organisms are exothermic, releasing energy to sustain life. The breakdown of glucose during cellular respiration is an example, with a negative ΔH.
Practical Applications: Harnessing Exothermic Reactions
The understanding of exothermic reactions and their negative ΔH values is crucial for various practical applications:
- Energy generation: Power plants rely on exothermic combustion reactions to generate electricity.
- Heating and cooling: Exothermic reactions are used in hand warmers and other heating devices.
- Industrial processes: Many industrial processes rely on the heat generated from exothermic reactions.
- Chemical synthesis: Exothermic reactions can drive desired chemical transformations.
Conclusion: The Unwavering Connection
In summary, the statement "Is a negative delta H exothermic?" has only one correct answer: yes. A negative enthalpy change (ΔH) is the definitive indicator of an exothermic process, where energy is released from the system to the surroundings in the form of heat. This fundamental relationship is essential in chemistry, thermodynamics, and numerous practical applications, making its understanding paramount for scientists, engineers, and anyone interested in the world of energy transformations. The connection is not merely correlative; it's a direct and fundamental principle governing energy changes in the universe.
Latest Posts
Latest Posts
-
Equations For Cellular Respiration And Photosynthesis
Mar 17, 2025
-
A Reflex That Causes Muscle Relaxation And Lengthening In Response
Mar 17, 2025
-
Compare And Contrast Magnification And Resolution
Mar 17, 2025
-
Effective Nuclear Charge Vs Nuclear Charge
Mar 17, 2025
-
What Is The Opposite Of Sublimation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is A Negative Delta H Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
