Is An Ionic Bond Between A Metal And Nonmetal
Muz Play
Mar 18, 2025 · 6 min read
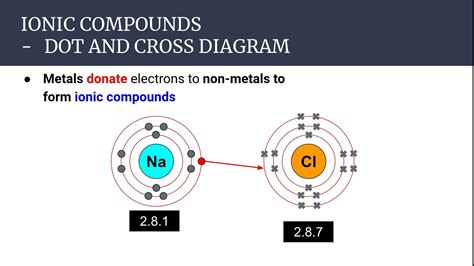
Table of Contents
Is an Ionic Bond Between a Metal and Nonmetal? A Deep Dive into Chemical Bonding
Ionic bonds are a fundamental concept in chemistry, crucial for understanding the properties and behavior of a vast array of substances. The simple answer to the question posed in the title is a resounding yes. Ionic bonds almost exclusively form between a metal and a nonmetal. This article will delve deep into the intricacies of ionic bonding, exploring the driving forces behind its formation, the properties of ionic compounds, and providing numerous examples to solidify your understanding.
Understanding the Fundamentals: Metals and Nonmetals
Before diving into the specifics of ionic bonding, it's crucial to understand the characteristics of metals and nonmetals. These characteristics dictate their behavior in chemical reactions and ultimately determine the formation of ionic bonds.
Metals: Electron Donors
Metals are characterized by their low electronegativity. This means they have a relatively weak hold on their valence electrons (the electrons in the outermost shell). They readily lose these electrons to achieve a stable electron configuration, often resembling the nearest noble gas. This electron loss results in the formation of positively charged ions, also known as cations. Think of metals as generous electron donors, happily giving up their electrons to achieve stability. Examples include sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca).
Nonmetals: Electron Acceptors
Nonmetals, on the other hand, exhibit high electronegativity. They possess a strong attraction for electrons and readily gain electrons to achieve a stable electron configuration, also often resembling a noble gas. This electron gain results in the formation of negatively charged ions, known as anions. Nonmetals can be seen as eager electron acceptors, readily grabbing electrons to complete their outermost electron shell. Examples include chlorine (Cl), oxygen (O), fluorine (F), and bromine (Br).
The Formation of an Ionic Bond: Electrostatic Attraction
The formation of an ionic bond is driven by the electrostatic attraction between oppositely charged ions. When a metal atom (with low electronegativity) encounters a nonmetal atom (with high electronegativity), the metal atom readily donates one or more electrons to the nonmetal atom. This transfer of electrons leads to the formation of a cation (positive ion) and an anion (negative ion). The strong electrostatic force of attraction between the positively charged cation and the negatively charged anion constitutes the ionic bond.
This process can be visualized with a simple example: the formation of sodium chloride (NaCl), common table salt. Sodium (Na), a metal, readily loses one electron to achieve a stable electron configuration, forming a Na⁺ cation. Chlorine (Cl), a nonmetal, readily gains one electron to achieve a stable electron configuration, forming a Cl⁻ anion. The electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic bond, resulting in the formation of the ionic compound NaCl.
Properties of Ionic Compounds: A Reflection of Strong Bonds
The strong electrostatic forces within ionic compounds result in several characteristic properties:
- High Melting and Boiling Points: The strong attractive forces between ions require a significant amount of energy to overcome, resulting in high melting and boiling points.
- Crystalline Structure: Ionic compounds typically form crystalline structures, with ions arranged in a highly ordered three-dimensional lattice. This arrangement maximizes the electrostatic attraction between the oppositely charged ions.
- Hardness and Brittleness: While generally hard, ionic compounds are also brittle. The application of force can cause the alignment of like charges, leading to repulsion and fracture.
- Solubility in Polar Solvents: Ionic compounds are often soluble in polar solvents like water, where the polar water molecules can effectively interact with and separate the ions.
- Electrical Conductivity: Ionic compounds typically conduct electricity when molten (liquid) or dissolved in a solution, as the ions become mobile and can carry an electric current. In their solid state, however, they are poor conductors because the ions are held rigidly in the crystal lattice.
Examples of Ionic Compounds: A Diverse Range
Numerous examples showcase the ubiquitous nature of ionic bonds in a variety of compounds:
- Sodium Chloride (NaCl): Table salt, formed by the interaction of sodium (metal) and chlorine (nonmetal).
- Magnesium Oxide (MgO): A white crystalline solid used in various applications, formed from magnesium (metal) and oxygen (nonmetal).
- Potassium Chloride (KCl): Used as a salt substitute and in various industrial applications, formed from potassium (metal) and chlorine (nonmetal).
- Calcium Carbonate (CaCO₃): The main component of limestone and marble, formed from calcium (metal) and carbonate (polyatomic nonmetal ion).
- Aluminum Oxide (Al₂O₃): A hard, refractory material used in various industrial processes, formed from aluminum (metal) and oxygen (nonmetal).
- Iron(III) Oxide (Fe₂O₃): A common iron ore, rust, formed from iron (metal) and oxygen (nonmetal).
Beyond the Basics: Factors Influencing Ionic Bond Strength
While the fundamental principle of an ionic bond is the electrostatic attraction between oppositely charged ions, several factors influence the strength of this bond:
- Charge of the Ions: The greater the charge of the ions, the stronger the electrostatic attraction and hence the stronger the ionic bond. For instance, the bond in MgO (Mg²⁺ and O²⁻) is stronger than the bond in NaCl (Na⁺ and Cl⁻).
- Size of the Ions: Smaller ions lead to stronger ionic bonds because the distance between the oppositely charged nuclei is smaller, resulting in a stronger electrostatic attraction.
- Lattice Energy: This is the energy released when gaseous ions combine to form a solid ionic crystal. Higher lattice energy implies a stronger ionic bond.
Exceptions and nuances: A Complex World
While the metal-nonmetal interaction is the dominant factor in ionic bond formation, some exceptions and nuances exist. Covalent character can be introduced in ionic bonds if the electronegativity difference between the metal and nonmetal isn't significant. The degree of ionic character can be predicted using concepts like electronegativity difference. Highly polar covalent bonds might exhibit ionic behavior to a certain degree.
Conclusion: A Foundation of Chemistry
The formation of an ionic bond between a metal and a nonmetal is a cornerstone of chemical bonding. Understanding the interplay between metallic and nonmetallic properties, the driving forces of electrostatic attraction, and the resulting properties of ionic compounds is essential for comprehending a vast array of chemical phenomena. From the formation of simple salts to the complex structures of minerals and biological systems, ionic bonds play a vital role in shaping our world. Further exploration into the intricacies of chemical bonding, including factors influencing bond strength and exceptions to the general rules, will enrich your understanding of this fundamental concept in chemistry. The insights provided here serve as a foundation for deeper dives into the fascinating realm of chemical interactions.
Latest Posts
Latest Posts
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is An Ionic Bond Between A Metal And Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
