Is Boiling Point Intensive Or Extensive
Muz Play
Mar 15, 2025 · 6 min read
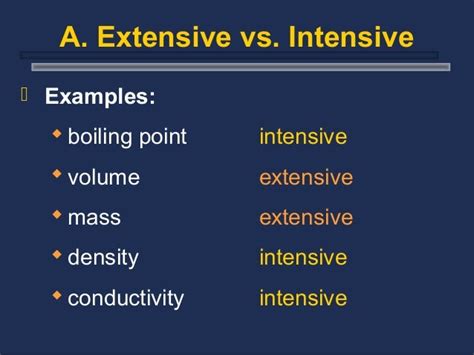
Table of Contents
Is Boiling Point Intensive or Extensive? Understanding Properties of Matter
The question of whether boiling point is an intensive or extensive property is a fundamental concept in chemistry and physics. Understanding this distinction is crucial for grasping the behavior of matter and its various states. This comprehensive article will delve deep into the definitions of intensive and extensive properties, clearly explain why boiling point is classified as an intensive property, and explore related concepts to solidify your understanding.
Intensive vs. Extensive Properties: A Clear Definition
Before we classify boiling point, let's establish a clear understanding of the difference between intensive and extensive properties. These terms describe how a property changes with the amount of matter present.
Intensive Properties: These properties are independent of the amount of substance. They remain constant regardless of whether you have a small sample or a large quantity. Think of it like this: if you divide a sample in half, the intensive properties of each half remain the same as the original sample. Examples include:
- Temperature: The temperature of a cup of water is the same as the temperature of a swimming pool full of water, assuming they are both at the same temperature.
- Density: The density of gold remains the same whether you have a gold nugget or a gold bar.
- Boiling point: This is the key focus of our article and will be thoroughly discussed.
- Melting point: Similar to boiling point, it remains constant regardless of sample size.
- Pressure: The pressure exerted by a gas is independent of the amount of gas (at a given temperature and volume).
- Refractive index: A measure of how light bends when passing through a material.
Extensive Properties: These properties are dependent on the amount of substance. If you divide a sample in half, the extensive property of each half will be half the value of the original sample. Examples include:
- Mass: The mass of 1 kg of water is twice the mass of 0.5 kg of water.
- Volume: The volume of a liter of water is twice the volume of 0.5 liters of water.
- Length: A 2-meter long rod is twice as long as a 1-meter long rod.
- Heat capacity: The amount of heat required to raise the temperature of a substance is proportional to its mass.
- Energy: The total energy of a system is dependent on the amount of matter.
Why Boiling Point is an Intensive Property
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the external pressure. Crucially, this temperature is independent of the amount of liquid present.
Imagine you have a small beaker containing 100ml of water and a large pot containing 1 liter of water, both at standard atmospheric pressure. They will both start boiling at approximately 100°C (212°F). Adding more water to the pot doesn't change the temperature at which it boils. This demonstrates that the boiling point is an intensive property. The boiling point is an inherent characteristic of the substance, determined by its intermolecular forces and external pressure.
The Role of External Pressure
It's important to note that while the boiling point is intensive, the external pressure significantly influences the boiling point. A lower external pressure will result in a lower boiling point, and vice versa. This is why water boils at a lower temperature at high altitudes where the atmospheric pressure is reduced. However, the boiling point at a specific pressure remains an intensive property. The relationship between boiling point and pressure is described by the Clausius-Clapeyron equation.
Impurities and Boiling Point
The presence of impurities can also affect the boiling point. However, this effect is generally small for dilute solutions. The addition of a non-volatile solute (like salt) to water will slightly elevate the boiling point, a phenomenon known as boiling point elevation. This elevation is a colligative property, meaning it depends on the concentration of solute particles rather than the identity of the solute. Nevertheless, this effect does not change the fundamental nature of the boiling point as an intensive property. The boiling point is still characteristic of the solution as a whole, and not dependent on the mass of the solution.
Further Exploring Intensive Properties: Applications and Examples
Understanding intensive properties is vital in various scientific and engineering disciplines. Their constancy regardless of sample size makes them incredibly useful for:
-
Material Identification: Intensive properties like melting point, boiling point, density, and refractive index are used extensively to identify unknown substances. These properties are often tabulated and compared against known values.
-
Chemical Reactions: Monitoring intensive properties during a chemical reaction can provide valuable insight into the reaction's progress and the properties of the products formed. For instance, tracking the temperature change during an exothermic reaction helps determine the reaction rate and energy released.
-
Phase Transitions: Intensive properties are crucial in defining phase transitions. The boiling point precisely marks the transition from liquid to gas phase, while the melting point marks the transition from solid to liquid.
-
Thermodynamics: Intensive properties play a fundamental role in thermodynamic calculations, often appearing in equations related to state functions and equilibrium conditions.
Contrast with Extensive Properties in Practical Applications
The difference between intensive and extensive properties is crucial in scaling up processes. For example, if you're designing a chemical reactor, you need to consider both types of properties. The amount of reactants (extensive) dictates the overall yield, but the reaction temperature (intensive) determines the reaction rate and product quality. Understanding this interplay is key to optimizing the process.
Common Misconceptions about Boiling Point
Several misunderstandings often arise regarding the boiling point's classification. Let's address some of them:
-
Confusion with heat energy: While you need to supply heat energy to raise a substance's temperature to its boiling point, the boiling point itself is not a measure of energy. The amount of heat required is an extensive property (heat capacity), but the temperature at which boiling occurs is intensive.
-
Impact of container size: The size or shape of the container doesn't directly affect the boiling point. While larger containers might take longer to reach the boiling point due to a higher heat capacity, the temperature at which boiling begins remains unchanged.
-
Mixing substances: When you mix substances, the boiling point of the resulting mixture will be different from the boiling points of the individual components. However, this boiling point is still an intensive property of the mixture.
Conclusion: Boiling Point Remains Intensive
In conclusion, the boiling point is definitively classified as an intensive property. Its value remains constant regardless of the amount of substance present, making it a useful characteristic for identifying and understanding matter. While external factors like pressure and the presence of impurities can affect the boiling point, this does not alter its fundamental nature as an intensive property. Understanding this distinction between intensive and extensive properties is vital for a complete understanding of the physical and chemical behavior of matter. This knowledge is instrumental across various scientific disciplines and engineering applications. By grasping this core concept, you establish a strong foundation for more advanced studies in chemistry, physics, and related fields.
Latest Posts
Latest Posts
-
An Atom Becomes A Positive Ion By Gaining An Electron
May 09, 2025
-
Atomic Number Of An Atom Reveals The Number Of
May 09, 2025
-
How To Check An Inverse Function
May 09, 2025
-
An Organic Compound That Contains Instructions For Proteins
May 09, 2025
-
The Most Prominent Organelle In A Eukaryotic Cell Is The
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Point Intensive Or Extensive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.