Is Boron An Exception To The Octet Rule
Muz Play
Apr 07, 2025 · 6 min read
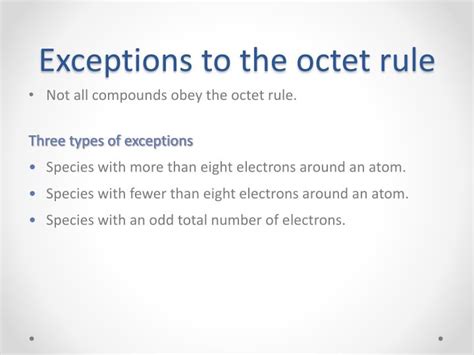
Table of Contents
Is Boron an Exception to the Octet Rule?
Boron, a metalloid element residing in group 13 of the periodic table, presents a fascinating case study in chemical bonding. While the octet rule, which dictates that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons, serves as a useful guideline, boron frequently defies this principle. This article delves deep into the reasons behind boron's exceptional behavior, exploring its bonding characteristics, molecular structures, and the underlying quantum mechanical principles at play.
Understanding the Octet Rule and its Limitations
The octet rule is a cornerstone of basic chemistry, explaining the stability of noble gases and influencing the bonding behavior of many elements. This rule stems from the fact that a complete valence shell with eight electrons (or two for hydrogen and helium) provides maximum stability due to a filled s and p subshell. Atoms strive to achieve this stable electron configuration through chemical bonding.
However, the octet rule is not a universally applicable law; it's more of a helpful guideline. Several elements, particularly those in the second period and beyond, exhibit exceptions. These exceptions are often due to factors such as the availability of d orbitals, the electronegativity of the bonded atoms, and the overall energy considerations of the resulting molecule.
Boron's Electronic Configuration: The Root of the Exception
Boron possesses an electronic configuration of 1s²2s²2p¹. This means it has only three valence electrons. To achieve a complete octet, boron would need to gain five more electrons, a highly unfavorable process. Instead, boron often forms compounds with fewer than eight electrons in its valence shell, typically forming three covalent bonds.
Electron Deficient Compounds: The Hallmark of Boron
This tendency to form fewer than four bonds results in electron-deficient compounds. These compounds are characterized by having fewer electrons than required for a complete octet for all atoms involved. This "electron deficiency" is a defining characteristic that sets boron apart from many other elements.
Common Examples of Boron's Octet Rule Violation
Several common boron compounds serve as compelling examples of its ability to violate the octet rule. Let's explore a few:
1. Boron Trifluoride (BF₃)
Boron trifluoride, a colorless toxic gas, is a classic example. Boron forms three covalent bonds with three fluorine atoms. However, this leaves boron with only six valence electrons, two short of a complete octet. The molecule is electron-deficient, and it actively seeks to gain more electrons. This is reflected in its strong Lewis acidity – its ability to accept electron pairs from Lewis bases.
2. Boron Trichloride (BCl₃)
Similar to BF₃, boron trichloride (BCl₃) also demonstrates boron's tendency towards incomplete octets. It exhibits similar electron-deficient nature and Lewis acidity. However, the B-Cl bond is weaker than the B-F bond due to the lower electronegativity of chlorine compared to fluorine.
3. Diborane (B₂H₆)
Diborane (B₂H₆) represents an even more intriguing example. This molecule contains two boron atoms, each forming three bonds, but only possessing six electrons. To explain its structure, the concept of three-center two-electron bonds is introduced. In diborane, two hydrogen atoms bridge between the two boron atoms, forming these unique bonds where three atoms (two boron and one hydrogen) share two electrons. These three-center, two-electron bonds are responsible for the molecule's stability, even with boron's incomplete octet.
Why Doesn't Boron Always Follow the Octet Rule?
Several factors contribute to boron's tendency to form compounds with less than eight valence electrons:
-
High Ionization Energy: Removing additional electrons from boron to reach an octet requires a significant amount of energy. The energy cost is far too high to be energetically favorable.
-
Small Atomic Size: Boron’s small size limits the number of atoms it can effectively bond with. Steric hindrance becomes a significant factor when attempting to fit more atoms around a small boron atom.
-
Relatively Low Electronegativity: Boron's relatively low electronegativity makes it less likely to attract and strongly hold extra electrons.
-
Energetic Favorability of 3 Bonds: The formation of three covalent bonds is energetically more favorable for boron than attempting to achieve a complete octet.
Beyond the Octet Rule: Exploring Quantum Mechanical Explanations
The octet rule is a simplified model. A more accurate description requires a quantum mechanical approach, which considers the molecular orbitals and their energies. Boron's behavior can be better understood by examining its hybrid orbitals. In BF₃, for instance, boron utilizes sp² hybridization, leading to a trigonal planar geometry. The three sp² hybrid orbitals each participate in a sigma bond with a fluorine atom, leaving an empty p orbital on the boron atom. This empty p orbital contributes to the molecule's Lewis acidity.
Boron's Role in Different Chemical Environments: A Deeper Dive
Boron's ability to disobey the octet rule extends its chemical versatility and makes it a crucial component in various chemical environments.
Boron in Organic Chemistry
Organoboron compounds play an increasingly important role in organic synthesis. The unique bonding characteristics of boron enable selective reactions and the formation of complex organic molecules. The stability of these compounds, despite their electron deficiency, is key to their widespread use in the field.
Boron in Materials Science
Boron is a vital element in various materials. Boron carbide (B₄C), for instance, is known for its exceptional hardness and is used in applications requiring high wear resistance. The bonding characteristics of boron contribute to the unique properties of these materials.
Boron in Biological Systems
Although less prominent than other elements, boron plays a subtle but significant role in certain biological systems. Its presence in some plants is known to enhance their growth and development. The precise mechanisms involved are still under investigation, but its unique chemical behavior is likely to play a vital role.
Conclusion: Boron's Exception as a Rule
Boron's deviation from the octet rule is not an anomaly; it reflects the limitations of simplified chemical models. Understanding boron's behavior necessitates considering its unique electronic configuration, atomic size, and the energetic factors influencing bond formation. Its ability to form electron-deficient compounds has led to the development of various synthetic pathways and novel materials. The three-center two-electron bonds found in diborane, for example, demonstrate how elements can achieve stability through innovative bonding arrangements beyond the conventional octet rule. In essence, boron's exceptional behavior underscores the beauty and complexity of chemical bonding, highlighting that while the octet rule serves as a useful guide, it does not encompass the entirety of chemical reality. Further research continues to uncover the intricacies of boron's chemical interactions, revealing its potential for further applications in various fields. The study of boron's exceptions to the octet rule highlights the ever-evolving nature of our understanding of chemical bonding and the diverse ways elements can achieve stability.
Latest Posts
Latest Posts
-
Conditional Statements That Are Always True Are Called
Apr 08, 2025
-
How Many Water Molecules Self Ionize In One Liter Of Water
Apr 08, 2025
-
Biology Chapter 1 The Study Of Life
Apr 08, 2025
-
Proof Of The Well Ordering Principle
Apr 08, 2025
-
Which State Of Matter Has Definite Shape
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Is Boron An Exception To The Octet Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.