Is Density And Specific Gravity The Same
Muz Play
Mar 30, 2025 · 6 min read
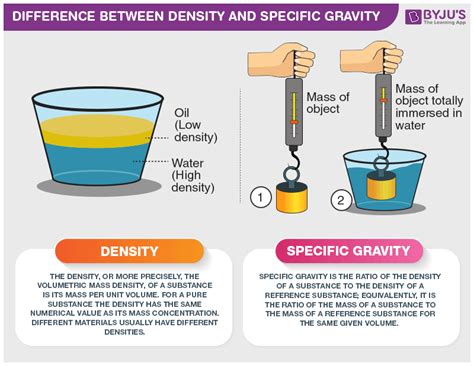
Table of Contents
Is Density and Specific Gravity the Same? Understanding the Subtle Differences
Density and specific gravity are two closely related concepts often used interchangeably, leading to confusion. While they both describe the concentration of mass within a substance, there's a crucial distinction: density is an absolute measure, while specific gravity is a relative measure. This article will delve deep into the definitions, calculations, applications, and subtle differences between these two important physical properties.
Defining Density: Mass per Unit Volume
Density, simply put, is the mass of a substance per unit volume. It quantifies how much matter is packed into a given space. A substance with high density has a large amount of mass crammed into a small volume, while a low-density substance has the same mass spread over a larger volume.
Formula for Density:
ρ = m/V
Where:
- ρ (rho) represents density
- m represents mass (typically in grams or kilograms)
- V represents volume (typically in cubic centimeters or cubic meters)
The SI unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also commonly used.
Examples of Density:
- Water: At 4°C, water has a density of approximately 1 g/cm³ or 1000 kg/m³. This is often used as a reference point for comparing the densities of other substances.
- Gold: Gold is a much denser material than water, with a density of approximately 19.3 g/cm³.
- Air: Air has a very low density, typically around 1.2 kg/m³ at sea level.
Understanding density is crucial in various fields, from material science and engineering to fluid mechanics and environmental science. For instance, the density of a material influences its strength, buoyancy, and behavior in different environments.
Defining Specific Gravity: Relative Density Comparison
Specific gravity, also known as relative density, is the ratio of the density of a substance to the density of a reference substance. The reference substance is usually water at 4°C (its maximum density). Therefore, specific gravity is a dimensionless quantity; it's a pure number without units.
Formula for Specific Gravity:
SG = ρ<sub>substance</sub> / ρ<sub>water</sub>
Where:
- SG represents specific gravity
- ρ<sub>substance</sub> represents the density of the substance
- ρ<sub>water</sub> represents the density of water at 4°C (approximately 1 g/cm³ or 1000 kg/m³)
Since specific gravity is a ratio, a value greater than 1 indicates that the substance is denser than water, while a value less than 1 indicates that it is less dense than water. A value of 1 means the substance has the same density as water.
Examples of Specific Gravity:
- Specific Gravity of Water: The specific gravity of water is, by definition, 1.
- Specific Gravity of Gold: Since gold has a density of approximately 19.3 g/cm³, its specific gravity is approximately 19.3.
- Specific Gravity of Air: The specific gravity of air is much less than 1, reflecting its lower density compared to water.
Specific gravity is widely used in various industries, including the mining and petroleum industries, for quick density comparisons. It's a convenient measure because it avoids the need to specify units.
Key Differences Between Density and Specific Gravity:
| Feature | Density | Specific Gravity |
|---|---|---|
| Type of Measure | Absolute measure | Relative measure |
| Units | kg/m³, g/cm³, etc. | Dimensionless (no units) |
| Reference | No reference substance | Typically water at 4°C |
| Value Range | Varies widely depending on the substance | Typically between 0 and 20 (depending on the substance) |
| Application | Wide range of applications | Often used for quick comparisons |
While the numerical value of specific gravity for a substance might seem similar to its density (if using g/cm³ for density and water as the reference), remember that they represent fundamentally different concepts. Density is an intrinsic property measuring mass per unit volume, while specific gravity compares the substance's density to a standard.
Practical Applications: Where Each Measure Shines
Both density and specific gravity find extensive use across various disciplines. However, their applications often differ based on the specific needs of the task:
Density's Diverse Applications:
- Material Science and Engineering: Density is crucial for selecting materials for specific applications. For example, a lightweight material with high strength is desirable for aerospace applications, while a high-density material might be needed for radiation shielding.
- Fluid Mechanics: Density plays a critical role in understanding fluid flow, buoyancy, and pressure distributions. Understanding the density of fluids is essential for designing pipelines, pumps, and other fluid-handling systems.
- Environmental Science: Density measurements are used to monitor water quality, assess pollution levels, and study sediment transport in rivers and oceans.
- Medical Applications: Density measurements are used in medical imaging techniques like bone densitometry (DEXA scans) to assess bone health.
Specific Gravity's Niche Applications:
- Gemology and Mining: Specific gravity is a simple and quick method for identifying gemstones and minerals. The specific gravity of a gem is a crucial characteristic used to determine authenticity.
- Petroleum Industry: Specific gravity is used extensively to determine the density of crude oil and its various products, influencing pricing and transportation.
- Food and Beverage Industry: Specific gravity is used to monitor the concentration of solutions, such as sugar content in syrups or the alcohol content in beverages.
- Battery Industry: Specific gravity is a critical parameter for monitoring the state of charge in lead-acid batteries.
Calculating Density and Specific Gravity: Practical Examples
Let's illustrate the calculations with some examples:
Example 1: Calculating Density
A block of wood has a mass of 500 grams and a volume of 625 cubic centimeters. What is its density?
ρ = m/V = 500 g / 625 cm³ = 0.8 g/cm³
Example 2: Calculating Specific Gravity
The density of ethanol is approximately 0.789 g/cm³. What is its specific gravity?
SG = ρ<sub>ethanol</sub> / ρ<sub>water</sub> = 0.789 g/cm³ / 1 g/cm³ = 0.789
Note that the units cancel out, leaving a dimensionless value.
Beyond the Basics: Temperature and Pressure Effects
It's important to note that both density and specific gravity are temperature and pressure-dependent. The density of most substances decreases with increasing temperature (due to thermal expansion) and increases with increasing pressure (due to compression). This is particularly important for gases, which are highly compressible. Therefore, when measuring density or specific gravity, it's crucial to control and report the temperature and pressure conditions. Standard conditions (such as standard temperature and pressure or STP) are often used for comparisons.
Conclusion: Choosing the Right Measure
Density and specific gravity, while related, serve different purposes. Density provides an absolute measure of mass concentration, while specific gravity offers a convenient relative comparison to a standard. The choice of which measure to use depends on the specific application and the information needed. Understanding the nuances of both concepts is essential for accurate measurements and insightful interpretations in various scientific and engineering fields. While seemingly simple, mastering the difference between these two concepts is crucial for accurate scientific work and engineering designs. Remember to always consider the context and the specific needs of your task when selecting the appropriate measure.
Latest Posts
Latest Posts
-
The Envelope Of A Virus Is Derived From The Hosts
Apr 01, 2025
-
Plant Cell In A Hypotonic Solution
Apr 01, 2025
-
What Is The Definition Of Form In Music
Apr 01, 2025
-
Staph Epidermidis Hemolysis On Blood Agar
Apr 01, 2025
-
Difference Between Autonomic And Somatic Nervous System
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Density And Specific Gravity The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
