Is Dipole Dipole Polar Or Nonpolar
Muz Play
Mar 28, 2025 · 5 min read
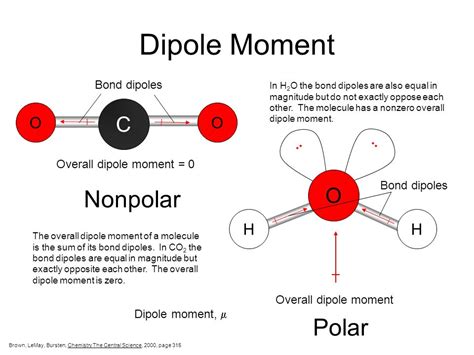
Table of Contents
Is Dipole-Dipole Interaction Polar or Nonpolar? Understanding Intermolecular Forces
The question of whether dipole-dipole interactions are polar or nonpolar is a bit of a trick question. The answer hinges on understanding what dipole-dipole interactions are and what properties define polar and nonpolar molecules. Let's delve into the details to clarify this concept.
Understanding Polarity and Intermolecular Forces
Before tackling dipole-dipole interactions, we need to define polarity and the different types of intermolecular forces.
Polar vs. Nonpolar Molecules
Molecular polarity arises from the uneven distribution of electron density within a molecule. This uneven distribution typically stems from differences in electronegativity between the atoms involved. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
-
Polar molecules: These molecules possess a permanent dipole moment, meaning there's a significant difference in electronegativity between atoms, leading to a partial positive charge (δ+) on one end and a partial negative charge (δ-) on the other. Think of water (H₂O): the oxygen atom is significantly more electronegative than the hydrogen atoms, pulling the electrons closer to itself and creating a polar molecule with a bent shape.
-
Nonpolar molecules: In nonpolar molecules, the electron density is relatively evenly distributed. This often occurs when the molecule is symmetrical, or when the electronegativity differences between atoms are negligible. Examples include methane (CH₄) and carbon dioxide (CO₂), although CO₂'s symmetry is crucial to its nonpolar nature despite having polar bonds.
Types of Intermolecular Forces
Intermolecular forces are the attractions between molecules. They are weaker than the intramolecular forces (bonds within a molecule), but they play a crucial role in determining the physical properties of substances like boiling point, melting point, and solubility. The main types include:
-
London Dispersion Forces (LDFs): These are the weakest intermolecular forces and are present in all molecules. They arise from temporary, instantaneous fluctuations in electron distribution, creating temporary dipoles. The strength of LDFs increases with the size and shape of the molecule.
-
Dipole-Dipole Forces: These forces occur between polar molecules. The positive end of one polar molecule is attracted to the negative end of another. They are stronger than LDFs.
-
Hydrogen Bonding: A special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. It's the strongest type of intermolecular force.
-
Ion-Dipole Forces: These forces occur between ions and polar molecules. The positive ion is attracted to the negative end of the polar molecule, and vice versa.
Dipole-Dipole Interactions: The Heart of the Matter
Now, let's directly address the question: Are dipole-dipole interactions polar or nonpolar?
The answer is unequivocally polar. Dipole-dipole interactions, by definition, only exist between polar molecules. These interactions are the result of the electrostatic attraction between the permanent dipoles of two polar molecules. The positive end of one dipole is attracted to the negative end of another, resulting in a net attractive force. This attraction is inherently a manifestation of the polarity of the individual molecules. You cannot have a dipole-dipole interaction without the pre-existing polarity in the molecules involved.
To illustrate this point, consider acetone (CH₃COCH₃). Acetone is a polar molecule due to the presence of a carbonyl group (C=O), which creates a significant dipole moment. Acetone molecules interact with each other through dipole-dipole forces – the partially positive carbon atom of one acetone molecule is attracted to the partially negative oxygen atom of another. This interaction is directly a consequence of the polar nature of the acetone molecule.
Examples and Contrasting with Nonpolar Interactions
Let's look at some examples to further clarify the distinction:
Polar Molecules and Dipole-Dipole Interactions:
-
Hydrogen Chloride (HCl): HCl is a polar molecule due to the electronegativity difference between hydrogen and chlorine. HCl molecules exhibit strong dipole-dipole interactions.
-
Ammonia (NH₃): Ammonia is a polar molecule with a pyramidal shape and a significant dipole moment. Its molecules interact through dipole-dipole forces, and these interactions are further enhanced by hydrogen bonding.
-
Ethanol (C₂H₅OH): Ethanol contains a hydroxyl (-OH) group, making it a polar molecule. It shows both dipole-dipole interactions and hydrogen bonding.
Nonpolar Molecules and London Dispersion Forces:
-
Methane (CH₄): Methane is a nonpolar molecule due to its symmetrical tetrahedral shape. Its molecules interact solely through weak London Dispersion Forces.
-
Carbon Tetrachloride (CCl₄): Similar to methane, CCl₄ is nonpolar and interacts only through London Dispersion Forces. Although carbon-chlorine bonds are polar, the symmetrical structure of the molecule cancels out the dipole moments.
-
Benzene (C₆H₆): Benzene is a nonpolar molecule; its symmetrical ring structure leads to an even distribution of electron density. Interactions between benzene molecules are based solely on London Dispersion Forces.
The Importance of Molecular Geometry:
It's crucial to remember that the geometry of a molecule plays a significant role in determining its polarity, and consequently, the type of intermolecular forces it exhibits. Even if individual bonds are polar, the overall molecular geometry can cancel out the dipole moments, resulting in a nonpolar molecule. This is evident in the case of CO₂ and CCl₄.
Consequences of Dipole-Dipole Interactions
The presence or absence of dipole-dipole interactions profoundly affects the physical properties of substances:
-
Boiling Point: Substances with dipole-dipole interactions generally have higher boiling points than those with only London Dispersion Forces. This is because the stronger dipole-dipole interactions require more energy to overcome during the phase transition from liquid to gas.
-
Solubility: Polar substances tend to dissolve better in polar solvents (like water) because of the favorable dipole-dipole interactions between solute and solvent molecules. Nonpolar substances dissolve better in nonpolar solvents due to London Dispersion Forces.
-
Melting Point: Similar to boiling point, dipole-dipole interactions contribute to higher melting points.
-
Viscosity: Liquids with stronger dipole-dipole interactions tend to have higher viscosities because the intermolecular attractions make the molecules more resistant to flow.
Conclusion: Dipole-Dipole Interactions and Polarity are Inseparable
In conclusion, dipole-dipole interactions are intrinsically linked to molecular polarity. They are a direct consequence of the uneven distribution of electron density within polar molecules. The strength of these interactions significantly influences a substance's physical properties. Understanding the relationship between molecular structure, polarity, and intermolecular forces is essential for predicting and explaining the behavior of different substances. The concept of dipole-dipole interaction is inherently polar, as it directly arises from and depends on the existence of a permanent dipole moment in the molecules involved.
Latest Posts
Latest Posts
-
Example Of A Line In A Poem
Mar 31, 2025
-
Interval Of Convergence Of A Taylor Series
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Dipole Dipole Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
