Is Hydrogen Bond Stronger Than Dipole Dipole
Muz Play
May 11, 2025 · 5 min read
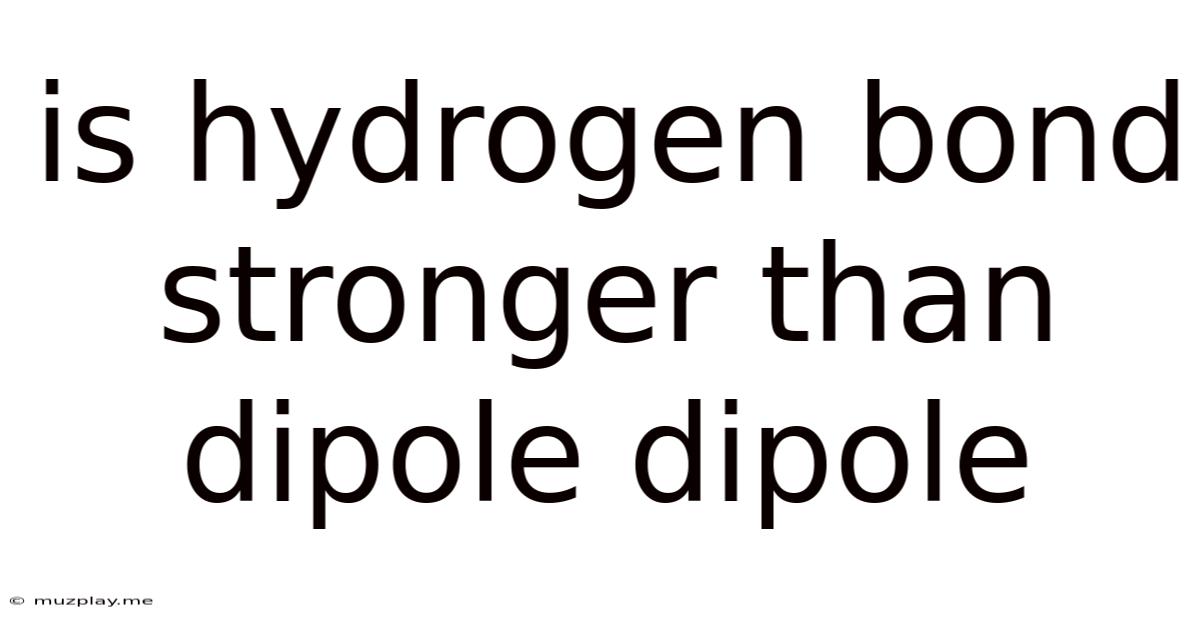
Table of Contents
Is a Hydrogen Bond Stronger Than a Dipole-Dipole Interaction? A Deep Dive
The question of whether a hydrogen bond is stronger than a dipole-dipole interaction is a common one in chemistry, and the answer isn't a simple yes or no. While hydrogen bonds are a type of dipole-dipole interaction, they are significantly stronger than most other dipole-dipole forces. This article will explore the nature of both hydrogen bonds and dipole-dipole interactions, comparing their strengths, and examining the factors that influence their relative magnitudes.
Understanding Dipole-Dipole Interactions
Dipole-dipole interactions occur between polar molecules. A polar molecule possesses a permanent dipole moment, meaning it has a slightly positive end (δ+) and a slightly negative end (δ−). This arises from an uneven distribution of electron density due to differences in electronegativity between the atoms within the molecule. The slightly positive end of one molecule is attracted to the slightly negative end of another, resulting in a weak electrostatic interaction. The strength of this interaction is directly proportional to the magnitude of the dipole moments involved and inversely proportional to the distance between the molecules.
Factors Affecting Dipole-Dipole Interaction Strength:
-
Magnitude of the dipole moment: Larger dipole moments lead to stronger dipole-dipole interactions. Molecules with larger differences in electronegativity between their atoms will have larger dipole moments.
-
Molecular shape: The shape of the molecule influences how effectively the dipoles can align and interact. Linear molecules often exhibit stronger dipole-dipole interactions than more spherical molecules because the dipoles can align more efficiently.
-
Distance between molecules: As the distance between molecules increases, the strength of the dipole-dipole interaction decreases rapidly.
Delving into Hydrogen Bonds: A Special Case of Dipole-Dipole Interactions
Hydrogen bonds are a specific type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (typically fluorine, oxygen, or nitrogen) is attracted to another electronegative atom in a different molecule or a different part of the same molecule. This strong attraction arises from the unique properties of hydrogen.
The Uniqueness of Hydrogen Bonds:
-
High electronegativity difference: The significant electronegativity difference between hydrogen and the electronegative atom (F, O, or N) leads to a highly polarized bond. The hydrogen atom carries a significant partial positive charge (δ+), making it strongly attracted to the lone pairs of electrons on the electronegative atom in another molecule.
-
Small size of hydrogen: The small size of the hydrogen atom allows for a close approach between the partially positive hydrogen and the electronegative atom, resulting in a stronger electrostatic interaction.
-
Stronger than typical dipole-dipole interactions: Due to the combined effects of high electronegativity difference and small size, hydrogen bonds are considerably stronger than most other dipole-dipole interactions. Their strength is typically in the range of 5-30 kJ/mol, while typical dipole-dipole interactions are much weaker, usually less than 10 kJ/mol.
Examples of Hydrogen Bonding:
Hydrogen bonds play crucial roles in numerous biological and chemical systems:
-
Water: The strong hydrogen bonds between water molecules are responsible for its high boiling point, surface tension, and solvent properties.
-
Proteins: Hydrogen bonds stabilize the secondary structure (alpha-helices and beta-sheets) of proteins.
-
DNA: Hydrogen bonds hold together the two strands of the DNA double helix.
-
Carbohydrates: Hydrogen bonds contribute to the structure and function of carbohydrates.
Comparing Strengths: Hydrogen Bonds vs. Other Dipole-Dipole Interactions
While hydrogen bonds are a subset of dipole-dipole interactions, their strength significantly surpasses that of most other dipole-dipole forces. This difference is primarily attributed to the exceptionally high polarity of the O-H, N-H, and F-H bonds and the small size of the hydrogen atom. Other dipole-dipole interactions involve molecules with less pronounced polarity and larger atomic distances, leading to weaker interactions.
Here's a table summarizing the key differences:
| Feature | Hydrogen Bond | Typical Dipole-Dipole Interaction |
|---|---|---|
| Bond type | O-H, N-H, F-H | Any polar bond |
| Electronegativity difference | Very high | Moderate |
| Atom size | Hydrogen (small) | Larger atoms |
| Interaction strength | 5-30 kJ/mol | Usually less than 10 kJ/mol |
| Specificity | Highly specific; directional | Less specific; less directional |
Factors Influencing Hydrogen Bond Strength:
Several factors can influence the strength of a hydrogen bond:
-
Electronegativity of the acceptor atom: More electronegative acceptor atoms (e.g., fluorine) lead to stronger hydrogen bonds.
-
Steric hindrance: Bulky groups surrounding the hydrogen bond donor or acceptor can hinder the close approach of the atoms, weakening the bond.
-
Solvent effects: The presence of a solvent can influence the strength of hydrogen bonds by competing for hydrogen bonds or by altering the dielectric constant of the medium.
-
Temperature: Hydrogen bond strength typically decreases with increasing temperature due to increased molecular motion.
Conclusion: Hydrogen Bonds - Stronger Dipole-Dipole Interactions
While hydrogen bonds are indeed a type of dipole-dipole interaction, they are significantly stronger than most other dipole-dipole forces. This enhanced strength stems from the unique combination of a highly polarized bond, the small size of the hydrogen atom, and the strong electrostatic attraction between the partially positive hydrogen and the highly electronegative atom. The strength of hydrogen bonds is crucial for the structure and function of many biological molecules and plays a critical role in various chemical and physical phenomena. Therefore, while technically a subcategory, hydrogen bonds display markedly greater strength and distinctive properties compared to the broader category of general dipole-dipole interactions. Understanding this difference is vital for comprehending the behavior of many substances and systems in chemistry and biology. The nuances of strength variations due to factors like electronegativity, steric hindrance, and solvent effects only further underscore the importance of recognizing hydrogen bonding as a distinct, powerful intermolecular force.
Latest Posts
Related Post
Thank you for visiting our website which covers about Is Hydrogen Bond Stronger Than Dipole Dipole . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.