Is Hydrogen More Electronegative Than Oxygen
Muz Play
Apr 01, 2025 · 5 min read
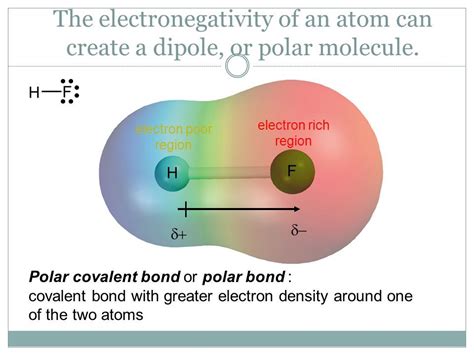
Table of Contents
Is Hydrogen More Electronegative Than Oxygen? A Deep Dive into Electronegativity
The question, "Is hydrogen more electronegative than oxygen?" has a straightforward answer: no. Oxygen is significantly more electronegative than hydrogen. However, understanding why requires a deeper dive into the concept of electronegativity and the factors that influence it. This article will explore electronegativity, compare the electronegativities of hydrogen and oxygen, examine the implications of this difference in various chemical contexts, and discuss related concepts like electropositivity and oxidation states.
Understanding Electronegativity
Electronegativity is a fundamental concept in chemistry that describes an atom's ability to attract shared electrons in a chemical bond. It's a relative property; we compare the electronegativity of one element to another. A higher electronegativity value indicates a stronger pull on electrons. Several scales exist to quantify electronegativity, the most common being the Pauling scale. On the Pauling scale, fluorine, the most electronegative element, is assigned a value of 4.0.
Several factors influence an atom's electronegativity:
-
Nuclear Charge: A higher nuclear charge (more protons in the nucleus) exerts a stronger attractive force on electrons.
-
Atomic Radius: A smaller atomic radius means the electrons are closer to the nucleus, experiencing a stronger pull.
-
Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge felt by the valence electrons.
-
Electron Configuration: Elements with nearly full valence shells tend to have higher electronegativities, as gaining an electron would complete the shell, a very stable configuration.
Comparing Hydrogen and Oxygen's Electronegativity
Hydrogen has an electronegativity of approximately 2.2 on the Pauling scale, while oxygen boasts a significantly higher value of approximately 3.44. This clear difference in electronegativity explains the nature of the bond between hydrogen and oxygen in water (H₂O) and other molecules containing both elements.
The Oxygen-Hydrogen Bond in Water
In the water molecule, the oxygen atom attracts the shared electrons in the O-H bonds more strongly than the hydrogen atoms. This unequal sharing of electrons leads to a polar covalent bond. Oxygen acquires a partial negative charge (δ-), while each hydrogen atom acquires a partial positive charge (δ+). This polarity is crucial to water's unique properties, including its high boiling point, excellent solvent capabilities, and its role as a vital component in biological systems.
Implications of the Electronegativity Difference
The difference in electronegativity between oxygen and hydrogen leads to several important chemical consequences:
-
Polarity of Molecules: As seen in water, a substantial electronegativity difference leads to polar molecules with distinct positive and negative poles. This polarity impacts the molecule's interactions with other molecules and its physical and chemical properties.
-
Bond Strength: While the electronegativity difference doesn't directly determine bond strength, it does influence the nature of the bond. A large difference can lead to ionic bonds, while a smaller difference results in covalent bonds with varying degrees of polarity. The O-H bond, while covalent, possesses a significant degree of polarity due to the electronegativity difference.
-
Reactivity: The high electronegativity of oxygen makes it a highly reactive element, readily forming bonds with many other elements. Hydrogen, while less electronegative, is also reactive, particularly in its diatomic form (H₂).
-
Oxidation States: The electronegativity difference is crucial in determining oxidation states in compounds. In water, oxygen generally exhibits an oxidation state of -2, while each hydrogen atom has an oxidation state of +1, reflecting the unequal sharing of electrons dictated by the electronegativity difference.
Electronegativity vs. Electropositivity
It's essential to distinguish electronegativity from electropositivity. Electropositivity refers to the tendency of an atom to lose electrons and form positive ions. Elements with low electronegativity are generally electropositive. While oxygen is highly electronegative, hydrogen demonstrates a degree of electropositivity, particularly when reacting with highly electronegative elements like oxygen, fluorine, or chlorine. This electropositivity contributes to the formation of the O-H bond in water and similar compounds.
Beyond Water: Other Examples
The electronegativity difference between oxygen and hydrogen is not unique to water. Numerous other molecules contain O-H bonds exhibiting similar polar characteristics. These include:
-
Alcohols: Alcohols (e.g., methanol, ethanol) contain hydroxyl (-OH) groups, where the oxygen atom attracts electrons more strongly than the hydrogen atom.
-
Carboxylic Acids: Carboxylic acids (e.g., acetic acid) contain carboxyl (-COOH) groups which have both a carbonyl (C=O) and a hydroxyl group, exhibiting significant polarity due to oxygen's electronegativity.
-
Hydrogen Peroxide (H₂O₂): This molecule contains an oxygen-oxygen bond and two O-H bonds. While the O-O bond is less polar than the O-H bonds, the high electronegativity of oxygen influences the overall molecular polarity.
In all these cases, the higher electronegativity of oxygen compared to hydrogen plays a critical role in determining the molecule's properties and reactivity.
Expanding the Understanding: Trends in Electronegativity
Understanding the periodic trends in electronegativity helps to predict the behavior of elements in various chemical reactions. Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom) in the periodic table. This trend is explained by the interplay of nuclear charge, atomic radius, and shielding effects discussed earlier. Oxygen, located in the top right of the periodic table, reflects this trend with its high electronegativity. Hydrogen, located in the top left, demonstrates a relatively low value reflecting its smaller nuclear charge and single proton.
Conclusion: Oxygen's Superior Electronegativity
In conclusion, oxygen is demonstrably more electronegative than hydrogen. This fundamental difference in electronegativity is responsible for the polar nature of the O-H bond found in water and many other vital molecules. This polarity significantly influences the physical and chemical properties of these molecules, impacting their behavior and roles in various chemical and biological processes. Understanding the concepts of electronegativity and electropositivity, and their impact on bond polarity, oxidation states, and molecular reactivity, is fundamental to a thorough grasp of chemical bonding and the properties of matter. The difference between the electronegativity of oxygen and hydrogen is not merely an abstract concept but a key factor in shaping the world around us.
Latest Posts
Latest Posts
-
Characteristics Of Emotional And Behavioral Disorders
Apr 02, 2025
-
Mercator Map Projection Advantages And Disadvantages
Apr 02, 2025
-
How To Set Up A Vacuum Filtration
Apr 02, 2025
-
Chemistry Structure And Properties Nivaldo J Tro
Apr 02, 2025
-
Identify Which Criteria Are Used To Characterize Bacterial Colonies
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Hydrogen More Electronegative Than Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
