Is Ionic Between Metal And Nonmetal
Muz Play
Apr 07, 2025 · 5 min read
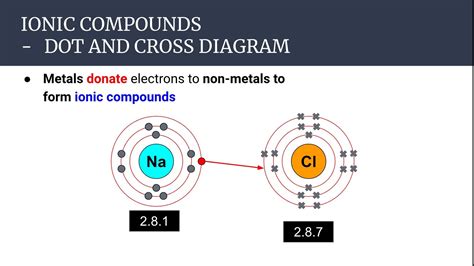
Table of Contents
Is Ionic Bonding Between Metal and Nonmetal? A Deep Dive into Ionic Compounds
Ionic bonding, a fundamental concept in chemistry, is a powerful force that governs the structure and properties of a vast array of materials. Understanding this type of bonding is crucial for comprehending the behavior of countless substances encountered in everyday life and advanced technologies. The simple answer to the question, "Is ionic bonding between metal and nonmetal?" is a resounding yes. However, the nuances of this interaction warrant a more detailed exploration. This article delves into the intricacies of ionic bonding, explaining its mechanism, characteristics, and exceptions, focusing on the crucial role played by the interaction between metals and nonmetals.
The Dance of Electrons: Understanding Ionic Bonds
At the heart of ionic bonding lies the transfer of electrons. Unlike covalent bonding, where atoms share electrons, ionic bonding involves a complete transfer of one or more electrons from one atom to another. This transfer creates ions: positively charged cations and negatively charged anions. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
The Role of Electronegativity
The driving force behind this electron transfer is the difference in electronegativity between the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Metals generally have low electronegativity, meaning they readily lose electrons. Nonmetals, conversely, have high electronegativity, readily accepting electrons. This significant difference in electronegativity is the key factor that facilitates the electron transfer and the formation of an ionic bond.
A simple analogy: Imagine a tug-of-war between a strong person (nonmetal, high electronegativity) and a weaker person (metal, low electronegativity). The strong person easily wins, pulling the "electron rope" (electrons) completely to their side.
Formation of Ions: A Closer Look
Let's examine the process with a classic example: the formation of sodium chloride (NaCl), common table salt. Sodium (Na), an alkali metal, has one valence electron. Chlorine (Cl), a halogen, needs one electron to complete its outer electron shell. Sodium, having a low electronegativity, readily loses its valence electron to become a positively charged sodium ion (Na⁺). Chlorine, with its high electronegativity, readily accepts this electron, becoming a negatively charged chloride ion (Cl⁻). The electrostatic attraction between the Na⁺ and Cl⁻ ions forms the ionic bond, creating the crystalline structure of NaCl.
Characteristics of Ionic Compounds
Ionic compounds, formed through ionic bonding between metals and nonmetals, exhibit several distinct characteristics:
-
Crystalline Structure: Ionic compounds form highly ordered, three-dimensional crystal lattices. This structure maximizes the electrostatic attraction between the oppositely charged ions, leading to a strong, stable compound. The arrangement of ions in the lattice depends on the size and charge of the ions involved.
-
High Melting and Boiling Points: The strong electrostatic forces between ions require significant energy to overcome, resulting in high melting and boiling points. This is a key distinguishing feature of ionic compounds compared to covalent compounds.
-
Hardness and Brittleness: Ionic crystals are generally hard due to the strong electrostatic interactions. However, they are also brittle because applying stress can cause the layers of ions to shift, leading to repulsion between ions of the same charge and causing the crystal to fracture.
-
Solubility in Polar Solvents: Many ionic compounds are soluble in polar solvents like water. Water molecules, being polar, can surround and interact with the ions, weakening the electrostatic forces holding the crystal lattice together and allowing the ions to dissolve.
-
Electrical Conductivity: Ionic compounds are generally poor conductors of electricity in their solid state because the ions are fixed in the crystal lattice. However, when molten (melted) or dissolved in a polar solvent, they become excellent conductors as the ions become mobile and can carry an electric current.
Exceptions and Nuances: When the Rules Bend
While the general rule of ionic bonding being between metals and nonmetals holds true for many compounds, there are exceptions and nuances to consider:
-
Polar Covalent Bonds: In some cases, the difference in electronegativity between a metal and a nonmetal may not be large enough to lead to a complete transfer of electrons. Instead, a polar covalent bond forms, where electrons are shared unequally. The resulting molecule will possess some ionic character, but it's not purely ionic.
-
Polyatomic Ions: Ionic compounds can also involve polyatomic ions, which are groups of atoms covalently bonded together that carry a net charge. For example, sodium nitrate (NaNO₃) contains the sodium ion (Na⁺) and the nitrate ion (NO₃⁻). The bonding within the nitrate ion is covalent, but the interaction between the Na⁺ and NO₃⁻ ions is ionic.
Beyond the Basics: Exploring Advanced Concepts
The understanding of ionic bonding extends far beyond the simple metal-nonmetal interaction. Several advanced concepts build upon this fundamental principle:
-
Lattice Energy: This refers to the energy released when gaseous ions combine to form a solid ionic crystal. The magnitude of lattice energy is a key indicator of the strength of the ionic bond. Larger charges and smaller ionic radii lead to higher lattice energies.
-
Born-Haber Cycle: This thermodynamic cycle allows the calculation of lattice energy indirectly using other experimentally measurable quantities like ionization energies, electron affinities, and enthalpy changes of formation. It provides a powerful tool to understand the energetics of ionic bond formation.
Applications of Ionic Compounds
Ionic compounds are ubiquitous and play essential roles in numerous applications:
- Table Salt (NaCl): A fundamental seasoning and preservative.
- Calcium Carbonate (CaCO₃): A major component of limestone, marble, and chalk; used in construction and as an antacid.
- Sodium Hydroxide (NaOH): A strong base used in various industrial processes.
- Potassium Chloride (KCl): Used as a fertilizer and in medical applications.
- Many Pharmaceuticals: Many drugs and medications utilize ionic compounds for their therapeutic effects.
Conclusion: A Foundation of Chemistry
The statement "ionic bonding is between metal and nonmetal" serves as a valuable rule of thumb. However, a deeper understanding necessitates considering electronegativity differences, the formation of ions, the resulting characteristics of ionic compounds, and the exceptions and nuances that can arise. Ionic bonding is a cornerstone of chemistry, underpinning the properties and behavior of a vast range of materials crucial to our daily lives and technological advancements. This fundamental concept is essential for any student or professional seeking a comprehensive understanding of chemistry and its applications. Further exploration of advanced concepts like lattice energy and the Born-Haber cycle provides an even richer appreciation of the complexity and importance of ionic bonding.
Latest Posts
Latest Posts
-
What Are Some Important Physics Concepts
Apr 09, 2025
-
What Are Three Properties Of Ionic Compounds
Apr 09, 2025
-
A Hybridization Experiment Involves Mating Blank
Apr 09, 2025
-
Lab One Diffusion And Osmosis Answers
Apr 09, 2025
-
What Is The Row Space Of A Matrix
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Is Ionic Between Metal And Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.