Is Metal Rusting A Chemical Or Physical Change
Muz Play
Mar 18, 2025 · 6 min read
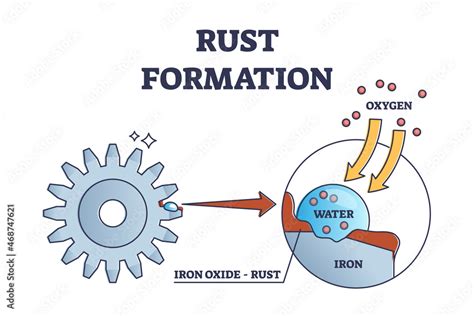
Table of Contents
Is Metal Rusting a Chemical or Physical Change? A Deep Dive
Rusting, that familiar orange-brown coating on iron and steel, is a process that affects billions of tons of metal globally every year. It's a significant concern for infrastructure, manufacturing, and countless everyday items. But beyond its practical implications, rusting presents a fascinating case study in chemistry. The core question: is rusting a chemical change or a physical change? The answer, unequivocally, is chemical. This article will explore why, delving into the scientific processes involved and dispelling common misconceptions.
Understanding Chemical vs. Physical Changes
Before we tackle the intricacies of rusting, let's establish a clear understanding of the difference between chemical and physical changes.
Physical Changes: A Change in Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of cutting paper, melting ice, or boiling water. The paper is still paper, the ice is still water, and the steam is still water – only their physical states have changed. These changes are often reversible.
Chemical Changes: A New Substance is Formed
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. These changes are often irreversible. Examples include burning wood (forming ash and gases), cooking an egg (changing the protein structure), or baking a cake (creating new compounds through chemical reactions).
The Chemistry of Rust: Oxidation and Reduction
Rusting, scientifically known as oxidation, is a prime example of a chemical change. It's a complex process involving a series of chemical reactions between iron (Fe) and oxygen (O<sub>2</sub>) in the presence of water (H<sub>2</sub>O) and often electrolytes like salt.
The Role of Oxygen: The Oxidizing Agent
Oxygen acts as an oxidizing agent, meaning it accepts electrons from another substance. In the case of rusting, oxygen readily accepts electrons from iron atoms. This electron transfer is the cornerstone of the chemical reaction. The iron atoms, having lost electrons, become positively charged ions (Fe<sup>2+</sup> or Fe<sup>3+</sup>).
The Role of Water: Facilitating the Reaction
Water plays a crucial role, acting as a medium for the reaction. It doesn't directly participate in the electron transfer but facilitates the movement of ions and dissolves the iron oxide that forms. The presence of dissolved salts in the water, such as those found in seawater or road salt, accelerates the rusting process by increasing the conductivity of the solution and speeding up ion movement.
The Formation of Iron Oxide: The Rust Itself
The positively charged iron ions react with hydroxide ions (OH<sup>-</sup>) from the water to form various iron oxides and hydroxides. The most common form is iron(III) oxide, Fe<sub>2</sub>O<sub>3</sub>, which is the reddish-brown substance we recognize as rust. The exact composition of rust can vary depending on the conditions, including the amount of water and oxygen present, as well as the pH of the environment.
Evidence that Rusting is a Chemical Change
Several key observations confirm that rusting is a chemical change, not a physical one:
- Irreversibility: Once iron has rusted, it's virtually impossible to simply reverse the process and recover the original iron metal. You can't "un-rust" an object; the chemical transformation is permanent.
- Color Change: The significant color change from the silvery-grey of iron to the reddish-brown of rust is a clear indicator of a new substance formation. Physical changes typically involve no color change, or only subtle alterations.
- Formation of a New Substance: The formation of iron oxide (rust) is a new substance with distinct chemical properties from the original iron. It has different physical properties, such as its color, texture, and solubility.
- Mass Change: While subtle, there's a measurable increase in mass during rusting. This increase is due to the addition of oxygen atoms to the iron atoms, resulting in a heavier iron oxide compound. Physical changes typically do not result in a significant mass change.
- Energy Changes: Rusting is an exothermic process, meaning it releases heat. This is a characteristic of many chemical reactions.
Factors Affecting the Rate of Rusting
Several factors influence the rate at which rusting occurs:
- Exposure to Oxygen and Water: The higher the concentration of oxygen and water, the faster the rusting process. Completely submerged iron may actually rust slower than iron exposed to both air and water due to the formation of a protective layer of rust.
- Temperature: Higher temperatures generally increase the rate of chemical reactions, including rusting.
- pH: Acidic environments accelerate rusting, while alkaline environments tend to slow it down. This explains why corrosion is often faster in acidic rain.
- Presence of Electrolytes: As previously mentioned, salts and other electrolytes increase the conductivity of the water, dramatically speeding up the rusting process. This is why saltwater environments are particularly corrosive to iron.
- Surface Area: A larger surface area of iron exposed to oxygen and water will rust faster than a smaller surface area. This is why iron powder rusts much faster than a solid iron block.
Preventing Rust: Protection Strategies
Because rusting is a costly and damaging process, considerable effort is put into preventing or slowing it down. Common prevention strategies include:
- Coating: Applying protective coatings like paint, varnish, or plastic prevents direct contact between the iron and oxygen and water, effectively slowing or preventing rust.
- Galvanization: Coating iron with a layer of zinc (galvanizing) provides sacrificial protection. Zinc reacts with oxygen and water instead of the iron, preventing rust until the zinc coating is depleted.
- Alloying: Creating alloys of iron, such as stainless steel, changes the metal's properties, making it less susceptible to rust. The addition of chromium, for example, forms a protective chromium oxide layer that resists further oxidation.
- Inhibitors: Chemicals called corrosion inhibitors can be added to water or other solutions to slow down the rusting process.
Conclusion: Rusting – A Definitive Chemical Change
In conclusion, the rusting of iron is undeniably a chemical change. The formation of iron oxide, the color change, the irreversibility, the mass change, and the energy released all point conclusively to a chemical reaction. Understanding the chemistry of rusting is not just an academic exercise; it's essential for protecting valuable metal structures and improving the durability of countless products in our daily lives. By understanding the factors that influence rusting, we can develop more effective strategies for prevention and mitigation, safeguarding our infrastructure and preserving valuable resources. The seemingly simple process of rusting highlights the intricate and powerful nature of chemical reactions that shape our world.
Latest Posts
Latest Posts
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Metal Rusting A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
