Is Saltwater A Pure Substance Or A Mixture
Muz Play
Mar 18, 2025 · 6 min read
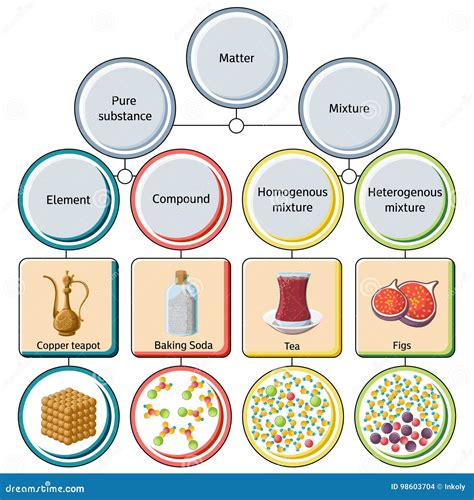
Table of Contents
Is Saltwater a Pure Substance or a Mixture? A Deep Dive into Chemical Composition
The question of whether saltwater is a pure substance or a mixture often arises in chemistry discussions. Understanding the difference between pure substances and mixtures is crucial for grasping fundamental chemical concepts. This comprehensive article will delve into the composition of saltwater, exploring its various components and definitively answering the question while also touching upon related concepts such as solutions, solvents, and solutes. We’ll also discuss the implications of this classification for various applications of saltwater.
Understanding Pure Substances and Mixtures
Before we classify saltwater, let's establish a clear understanding of the terms "pure substance" and "mixture."
Pure Substances: These are forms of matter with a fixed chemical composition and distinct properties. A pure substance cannot be separated into simpler components by physical methods (like filtration or distillation). Pure substances can be further classified into elements and compounds.
- Elements: These are substances made up of only one type of atom. Examples include oxygen (O), hydrogen (H), and gold (Au).
- Compounds: These are substances formed by the chemical combination of two or more elements in fixed proportions. The properties of a compound are different from the properties of its constituent elements. Examples include water (H₂O) and table salt (NaCl). Compounds can only be separated into their constituent elements through chemical means.
Mixtures: Mixtures are combinations of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties, and they can be separated by physical methods. Mixtures can be homogeneous or heterogeneous.
- Homogeneous Mixtures: These have a uniform composition throughout. You cannot visually distinguish the different components. Examples include saltwater, air, and sugar dissolved in water.
- Heterogeneous Mixtures: These have a non-uniform composition. You can visually distinguish the different components. Examples include sand and water, oil and water, and a salad.
The Composition of Saltwater
Saltwater, commonly found in oceans and seas, is a complex mixture, not a pure substance. Its primary components are:
- Water (H₂O): This is the solvent, making up the majority of saltwater. It is a compound, but as the major component of a mixture, it doesn't change the classification of the overall substance.
- Sodium Chloride (NaCl): This is the most abundant solute in saltwater, commonly known as table salt. It is an ionic compound formed by the electrostatic attraction between sodium (Na⁺) and chloride (Cl⁻) ions.
- Other Salts and Minerals: Seawater contains many other dissolved ions, including magnesium (Mg²⁺), calcium (Ca²⁺), potassium (K⁺), sulfate (SO₄²⁻), bicarbonate (HCO₃⁻), bromide (Br⁻), and many others in smaller quantities. These contribute to the overall salinity and properties of saltwater.
- Dissolved Gases: Seawater also contains dissolved gases like oxygen (O₂), nitrogen (N₂), and carbon dioxide (CO₂), crucial for marine life.
- Organic Matter: Various organic compounds, originating from decaying plants and animals, are also present in seawater.
- Suspended Particles: Depending on the location and environmental conditions, seawater can also contain suspended particles like sand, silt, and plankton.
Why Saltwater is a Mixture
The presence of multiple substances, each retaining its individual properties, firmly establishes saltwater as a mixture. The following points reinforce this classification:
- Variable Composition: The concentration of dissolved salts and other components in seawater varies geographically and depending on factors like river inflow, evaporation, and depth. A pure substance has a fixed composition.
- Separation by Physical Methods: The components of saltwater can be separated using techniques like evaporation, distillation, and filtration. Evaporation leaves behind the dissolved salts, while distillation can separate the water from other volatile components. These techniques are physical processes, not chemical reactions.
- Retention of Individual Properties: The properties of saltwater are a blend of the properties of its components. For example, the salty taste comes from the dissolved sodium chloride, while the liquid nature is due to the water. The individual components retain some of their intrinsic qualities even when mixed.
Saltwater as a Homogeneous Mixture
While saltwater contains various components, it's generally considered a homogeneous mixture. On a macroscopic scale, the components are evenly distributed throughout the solution. You cannot easily distinguish individual salt crystals or other dissolved substances by simply looking at seawater. However, microscopic analysis would reveal the individual ions and molecules.
Solutions, Solvents, and Solutes in Saltwater
Saltwater is a type of solution – a homogeneous mixture where one substance (the solute) is dissolved in another (the solvent). In saltwater:
- Solvent: Water (H₂O) is the solvent, the substance in which other substances dissolve. It's the primary component, determining the state of the solution (liquid in this case).
- Solutes: Sodium chloride (NaCl) and other dissolved salts and minerals are the solutes. They dissolve in the solvent, forming a homogeneous solution.
Implications of Saltwater's Classification
Understanding that saltwater is a mixture, not a pure substance, has significant implications in various fields:
- Oceanography: Oceanographers study the composition of seawater to understand marine ecosystems, ocean currents, and climate change. The varying salinity and mineral content are crucial aspects of this research.
- Desalination: Desalination processes are designed to separate water from salts and other impurities in seawater to provide fresh drinking water. The knowledge of saltwater's composition guides the development of effective desalination technologies.
- Marine Biology: Marine organisms have adapted to the specific salinity and chemical composition of seawater. Changes in saltwater composition due to pollution or climate change can have severe consequences for marine life.
- Chemistry and Material Science: Saltwater's properties are exploited in various chemical processes and material science applications. For example, it can be used as a solvent in certain reactions or as a component in electrochemical processes.
Common Misconceptions about Saltwater
Several misconceptions exist regarding the nature of saltwater:
- Saltwater is just salt and water: While salt and water are the main components, it's crucial to remember the presence of other dissolved minerals and gases.
- Saltwater is a compound because salt dissolves in water: The dissolution of salt in water is a physical process, not a chemical reaction. The chemical bonds within NaCl and H₂O remain intact.
- Saltwater is always the same: The composition of saltwater varies depending on location and environmental factors.
Conclusion
In conclusion, saltwater is unequivocally a mixture, specifically a homogeneous mixture. Its composition includes water as the solvent and various solutes such as sodium chloride, other salts, dissolved gases, and organic matter. The varying concentration of these components and the ability to separate them through physical processes definitively rule out the classification of saltwater as a pure substance. This understanding is crucial for various scientific disciplines and practical applications, from oceanography and desalination to marine biology and chemistry. Further research into the complexities of saltwater composition continues to reveal new insights into the dynamic nature of our oceans and the intricate interplay of their components.
Latest Posts
Latest Posts
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Saltwater A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
