Is Water A Reactant Or Product
Muz Play
Mar 31, 2025 · 6 min read
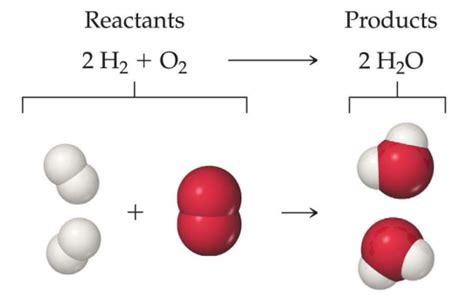
Table of Contents
Is Water a Reactant or Product? Understanding Water's Role in Chemical Reactions
Water, the elixir of life, plays a multifaceted role in the chemical world. It's not simply a passive bystander; its involvement in reactions is dynamic and crucial, sometimes as a reactant, other times as a product. Understanding whether water acts as a reactant or product in a given chemical equation is key to grasping the underlying chemistry. This comprehensive guide delves into the various scenarios where water participates, clarifying its role and highlighting the implications.
Water as a Reactant: Driving Chemical Change
In many reactions, water acts as a reactant, actively participating in the chemical transformation and undergoing a change itself. This involvement significantly influences the reaction's outcome and the products formed. Several key examples illustrate this:
1. Hydration Reactions: Adding Water to the Mix
Hydration reactions are a classic example where water acts as a reactant. These reactions involve the addition of water to a molecule, typically resulting in a new compound with altered properties. A common instance is the hydration of alkenes, where water adds across the double bond, yielding an alcohol.
Mechanism: The double bond in an alkene breaks, and the oxygen atom from the water molecule bonds to one carbon atom, while the hydrogen atom bonds to the other. This process, often catalyzed by an acid, transforms an unsaturated hydrocarbon into a saturated alcohol.
Example: The hydration of ethene (C₂H₄) produces ethanol (C₂H₅OH). Here, water is clearly a reactant, directly participating in the formation of the product.
Equation: C₂H₄ + H₂O → C₂H₅OH
2. Hydrolysis Reactions: Water as a Cleaving Agent
Hydrolysis reactions involve the breaking down of a molecule by the addition of a water molecule. Water acts as a reactant, cleaving a bond and producing two or more new molecules. This is prevalent in the breakdown of polymers like proteins and carbohydrates.
Mechanism: The water molecule attacks a bond within the polymer, breaking it and forming new bonds with the fragments. This process is crucial in digestion, where enzymes catalyze the hydrolysis of complex molecules into smaller, absorbable units.
Example: The hydrolysis of sucrose (table sugar) yields glucose and fructose. Water, as a reactant, participates directly in cleaving the glycosidic bond between the monosaccharides.
Equation: C₁₂H₂₂O₁₁ (Sucrose) + H₂O → C₆H₁₂O₆ (Glucose) + C₆H₁₂O₆ (Fructose)
3. Acid-Base Reactions: Water's Amphoteric Nature
Water exhibits amphoteric behavior, acting as both an acid and a base. In acid-base reactions, water can react with acids or bases, depending on the context.
Mechanism: When acting as a base, water accepts a proton (H⁺) from an acid, forming the hydronium ion (H₃O⁺). Conversely, when acting as an acid, water donates a proton to a base, forming the hydroxide ion (OH⁻).
Example: The reaction of water with hydrochloric acid (HCl) is a classic example. Water acts as a base, accepting a proton from HCl to form H₃O⁺ and Cl⁻.
Equation: HCl + H₂O → H₃O⁺ + Cl⁻
These examples underscore water's crucial role as a reactant in diverse chemical reactions, influencing reaction pathways and product formation. Its involvement is not merely incidental; it is fundamental to many essential chemical processes.
Water as a Product: The Result of Chemical Transformations
In other reactions, water emerges as a product, signifying the completion of a chemical transformation. The formation of water often indicates the creation of new bonds and the rearrangement of atoms.
1. Combustion Reactions: Water as a Byproduct
Combustion reactions, often involving the burning of organic compounds, typically yield carbon dioxide (CO₂) and water (H₂O) as products.
Mechanism: The oxidation of carbon and hydrogen atoms in the organic fuel leads to the formation of CO₂ and H₂O, respectively. The oxygen in the air acts as the oxidizing agent.
Example: The complete combustion of methane (CH₄) produces carbon dioxide and water.
Equation: CH₄ + 2O₂ → CO₂ + 2H₂O
2. Neutralization Reactions: Water as a Product of Acid-Base Reactions
Neutralization reactions between acids and bases result in the formation of water and a salt. Water is one of the primary products, indicating the neutralization of acidic and basic properties.
Mechanism: The proton (H⁺) from the acid combines with the hydroxide ion (OH⁻) from the base to form a water molecule. The remaining ions from the acid and base form the salt.
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) yields sodium chloride (NaCl) and water.
Equation: HCl + NaOH → NaCl + H₂O
3. Dehydration Reactions: Removing Water to Form New Bonds
Dehydration reactions are the opposite of hydration. They involve the removal of water from a molecule, often leading to the formation of a new bond between the remaining fragments. This process is crucial in the synthesis of many organic compounds.
Mechanism: A water molecule is eliminated from the reactant molecule, creating a double bond or a new ring structure. This is often facilitated by the use of dehydrating agents.
Example: The dehydration of alcohols can lead to the formation of alkenes. Water is a product removed from the alcohol molecule.
4. Esterification: Water is a byproduct of acid-alcohol reaction
In organic chemistry, esterification is the process of combining an acid and an alcohol to form an ester and water. Water is a byproduct of this condensation reaction.
Mechanism: The carboxyl group of the acid reacts with the hydroxyl group of the alcohol, releasing a water molecule and forming an ester linkage.
Example: The reaction of acetic acid and ethanol forms ethyl acetate and water.
Equation: CH₃COOH + CH₃CH₂OH → CH₃COOCH₂CH₃ + H₂O
These examples illustrate how water can be a product resulting from various chemical reactions, signifying a completion of the process and indicating the formation of new substances.
Factors Determining Water's Role
The role of water – as a reactant or product – depends on several factors:
-
The type of reaction: Certain reaction types, such as hydration and hydrolysis, inherently involve water as a reactant. Others, like combustion and neutralization, typically produce water as a product.
-
The reactants involved: The chemical nature of the reactants dictates whether water will participate as a reactant or form as a product.
-
Reaction conditions: Factors such as temperature, pressure, and the presence of catalysts can influence the reaction pathway and thus water's role.
Conclusion: Water's Dynamic Nature in Chemistry
In summary, water’s role in chemical reactions is far from passive. It exhibits remarkable versatility, acting as both a reactant and a product depending on the specific context. Understanding the conditions under which water participates in either capacity is crucial for comprehending a wide range of chemical processes, from the synthesis of organic compounds to the breakdown of complex biological molecules. Its amphoteric nature, its ability to act as both an acid and a base, further adds to its remarkable versatility in the chemical world. This multifaceted nature highlights the essential and dynamic role of water in chemistry. Its presence, either as a participant or a product, significantly impacts the outcome and characteristics of numerous chemical reactions. A deep understanding of its diverse roles is critical for anyone seeking to master the intricacies of chemical transformations.
Latest Posts
Latest Posts
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
-
In A Solution It Is Dissolving Medium
Apr 02, 2025
-
How To Find Moles Of Naoh Used In Titration
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Reactant Or Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
