Is Water Boiling A Chemical Change
Muz Play
Mar 16, 2025 · 4 min read
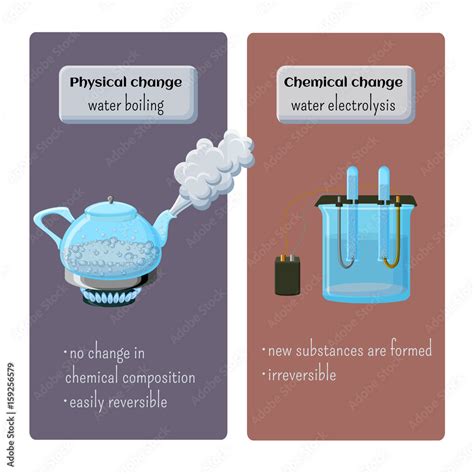
Table of Contents
- Is Water Boiling A Chemical Change
- Table of Contents
- Is Boiling Water a Chemical Change? Unraveling the Science Behind a Simple Process
- Understanding Chemical vs. Physical Changes
- Physical Changes: A Matter of Form, Not Substance
- Chemical Changes: A Transformation at the Molecular Level
- The Case of Boiling Water: A Detailed Examination
- The Molecular Dance: What Happens When Water Boils?
- No New Substances Formed: The Key Indicator
- Reversibility: A Strong Argument for a Physical Change
- Addressing Potential Counterarguments
- Dissociation of Water Molecules: A Minor Detail
- Decomposition at Extremely High Temperatures: A Different Scenario
- Conclusion: Boiling Water Remains a Physical Change
- Latest Posts
- Latest Posts
- Related Post
Is Boiling Water a Chemical Change? Unraveling the Science Behind a Simple Process
The question of whether boiling water constitutes a chemical change or a physical change is a surprisingly complex one, often sparking debate among students and enthusiasts of chemistry alike. While seemingly simple, the process of boiling water involves intricate interactions between water molecules and energy, requiring a nuanced understanding of chemical and physical transformations. This article delves deep into the science behind boiling water, examining the evidence and arguments to definitively answer this intriguing question.
Understanding Chemical vs. Physical Changes
Before diving into the specifics of boiling water, let's establish a clear understanding of the fundamental difference between chemical and physical changes.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think about cutting a piece of paper, melting an ice cube, or dissolving sugar in water. In each case, the substance's form changes, but the underlying chemical makeup remains the same. The paper is still paper, the ice is still water, and the sugar is still sugar, even if its state or location has altered. Physical changes are often reversible; you can freeze water back into ice, for instance.
Chemical Changes: A Transformation at the Molecular Level
A chemical change, also known as a chemical reaction, involves a fundamental alteration in the chemical composition of a substance. New substances with different properties are formed. Examples include burning wood (forming ash and gases), rusting iron (forming iron oxide), or baking a cake (complex chemical reactions between ingredients). Chemical changes are often irreversible or require significant energy to reverse.
The Case of Boiling Water: A Detailed Examination
Now, let's turn our attention to the central question: is boiling water a chemical change? The overwhelming scientific consensus points towards boiling water being a physical change.
The Molecular Dance: What Happens When Water Boils?
Water, in its liquid state, consists of water molecules (H₂O) held together by relatively weak intermolecular forces called hydrogen bonds. These bonds are responsible for water's unique properties, such as its high boiling point and surface tension. When heat is applied to water, the energy is absorbed by the water molecules, causing them to vibrate and move more rapidly.
As the temperature increases, the kinetic energy of the water molecules overcomes the hydrogen bonds holding them together. At the boiling point (100°C or 212°F at standard atmospheric pressure), these bonds break, and the water molecules transition from a liquid state to a gaseous state (steam or water vapor).
No New Substances Formed: The Key Indicator
Crucially, during the boiling process, no new chemical substances are formed. The water molecules remain H₂O molecules, even in the gaseous state. The change is merely in their state of matter and the way they interact with each other. This is the defining characteristic distinguishing a physical change from a chemical one. The steam produced is still chemically identical to the liquid water – just separated and moving more freely.
Reversibility: A Strong Argument for a Physical Change
The reversibility of boiling water further reinforces its classification as a physical change. Condensation, the process of steam turning back into liquid water, is readily achievable by simply cooling the steam. This demonstrates that the original substance is completely recoverable, a hallmark of a physical transformation.
Addressing Potential Counterarguments
While the evidence overwhelmingly supports boiling water as a physical change, some nuanced arguments might arise. Let's address them:
Dissociation of Water Molecules: A Minor Detail
It's true that a small fraction of water molecules undergo self-ionization, splitting into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). This process is an equilibrium reaction, meaning it occurs to a limited extent and is constantly reversed. The concentration of these ions in pure water is exceedingly low, and this minor dissociation doesn't constitute a significant chemical change impacting the overall nature of boiling water. The vast majority of water molecules remain as H₂O.
Decomposition at Extremely High Temperatures: A Different Scenario
At extremely high temperatures and under specific conditions, water molecules can decompose into hydrogen and oxygen gases (2H₂O → 2H₂ + O₂). This is a true chemical change, as entirely new substances are formed. However, this decomposition does not occur during typical boiling under normal atmospheric conditions. The decomposition of water requires significantly higher temperatures and often involves catalysts or electrical energy.
Conclusion: Boiling Water Remains a Physical Change
In conclusion, while nuanced points might be raised, the overwhelming scientific evidence supports the assertion that boiling water is a physical change, not a chemical change. No new substances are formed; the water molecules simply change their state of matter due to increased kinetic energy overcoming intermolecular forces. The process is reversible through condensation. The minor self-ionization of water and its decomposition at extreme temperatures are separate processes occurring under vastly different conditions, and do not affect the classification of boiling under standard circumstances. Understanding this distinction is crucial for grasping the fundamental principles of chemistry and the differences between physical and chemical transformations. The seemingly simple act of boiling water offers a rich opportunity to explore the fascinating world of molecular interactions and the states of matter.
Latest Posts
Latest Posts
-
Conversion Of Cartesian To Cylindrical Coordinates
Mar 17, 2025
-
Which Era Is Referred To As The Age Of Mammals
Mar 17, 2025
-
How To Analyze Mass Spectrometry Data
Mar 17, 2025
-
Difference Between Synapse And Button Terminal
Mar 17, 2025
-
What Seismic Wave Travels The Fastest
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Water Boiling A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
