Lewis Dot Diagram For 2 Individual Ions For Na
Muz Play
Apr 01, 2025 · 6 min read
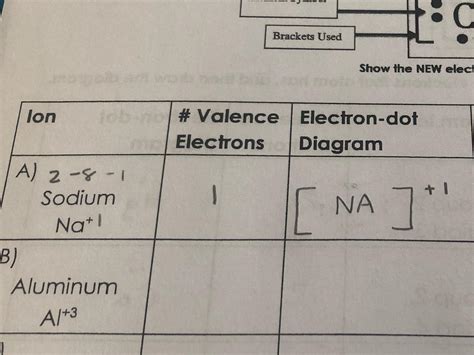
Table of Contents
Lewis Dot Diagrams for Individual Sodium Ions (Na⁺)
Understanding the electronic structure of atoms and ions is fundamental to chemistry. Lewis dot diagrams, also known as electron dot diagrams, provide a simple yet powerful visual representation of the valence electrons in an atom or ion. This article delves deep into the Lewis dot diagram for the sodium ion (Na⁺), exploring its formation, significance, and applications within the broader context of chemical bonding and reactivity.
Understanding Sodium and its Electronic Configuration
Sodium (Na), an alkali metal, is element number 11 on the periodic table. Its electronic configuration is 1s²2s²2p⁶3s¹. This means it has 11 electrons arranged in different energy levels or shells. The first shell (n=1) can hold a maximum of two electrons, the second shell (n=2) can hold up to eight electrons, and the third shell (n=3) can hold up to 18 electrons.
The outermost shell, which contains only one electron in sodium's case, is crucial for understanding its chemical behavior. Electrons in this outermost shell are called valence electrons. These electrons are involved in chemical bonding and determine the reactivity of an element.
Formation of the Sodium Ion (Na⁺)
Sodium is highly reactive because it only needs to lose one electron to achieve a stable electron configuration. This stable configuration, also known as a noble gas configuration, resembles the electron configuration of the nearest noble gas, Neon (Ne), which is 1s²2s²2p⁶. This drive to achieve stability is the driving force behind sodium's chemical reactivity.
By losing its single valence electron, sodium transforms into a positively charged ion, denoted as Na⁺. This process is called ionization. The removal of the electron leaves behind a positively charged nucleus with more protons than electrons.
Constructing the Lewis Dot Diagram for Na⁺
The Lewis dot diagram for a sodium atom shows the single valence electron as a dot around the symbol 'Na':
Na•
However, the Lewis dot diagram for the sodium ion (Na⁺) is significantly different. Since sodium loses its valence electron to become an ion, the Lewis dot diagram for Na⁺ simply shows the symbol 'Na' without any dots, representing the absence of valence electrons:
Na⁺
This is because the valence electron is no longer present. The ion now has a full outermost shell, mirroring the stable electron configuration of Neon.
Significance of the Lewis Dot Diagram for Na⁺
The simplicity of the Lewis dot diagram for Na⁺ belies its importance in understanding several key chemical concepts:
-
Ionic Bonding: The formation of Na⁺ highlights the concept of ionic bonding. Sodium readily loses its valence electron to form an ionic bond with a non-metal atom that readily gains electrons, such as chlorine (Cl). The resulting electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic compound sodium chloride (NaCl), commonly known as table salt.
-
Octet Rule: The transformation of Na to Na⁺ exemplifies the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. While sodium only has two shells after losing an electron, its outermost shell is full (a duet of electrons in the 2s shell) and thus stable. Exceptions to the octet rule exist, but it remains a useful guideline for understanding chemical bonding in many cases.
-
Predicting Chemical Reactivity: The Lewis dot diagram clearly shows sodium's strong tendency to lose an electron, making it highly reactive. This reactivity is a characteristic of alkali metals. The absence of dots in the Na⁺ diagram signifies the ion’s reduced reactivity compared to the neutral sodium atom.
-
Understanding Oxidation States: The formation of Na⁺ demonstrates the concept of oxidation state. Sodium has an oxidation state of +1 because it has lost one electron. The oxidation state reflects the apparent charge of an atom in a compound.
Comparison with Other Alkali Metals
Other alkali metals, such as lithium (Li), potassium (K), rubidium (Rb), and cesium (Cs), also readily lose one electron to form +1 ions. Their Lewis dot diagrams follow a similar pattern: the neutral atom shows one dot, while the ion shows no dots. This consistent behavior is a characteristic of the alkali metal group in the periodic table.
The size of the ions varies, however. As you go down the group, the ionic radius increases due to the addition of electron shells. This increase in size affects the properties of the compounds formed by these ions. For instance, the melting points of alkali metal halides decrease as the ionic radius increases.
Applications of Understanding Na⁺
Understanding the electronic structure of Na⁺ and its formation has far-reaching implications in various fields:
-
Electrochemistry: Na⁺ plays a crucial role in various electrochemical processes, including batteries and fuel cells. The movement of Na⁺ ions across membranes is vital in biological systems, contributing to nerve impulse transmission and muscle contraction.
-
Material Science: The properties of sodium-containing materials are heavily influenced by the presence of Na⁺ ions. Understanding the behavior of Na⁺ is essential in designing and developing new materials with specific properties.
-
Medicine and Biology: Sodium ions are vital electrolytes in biological systems, maintaining osmotic balance and contributing to the transmission of nerve impulses. Sodium channels and pumps in cell membranes regulate the movement of Na⁺ ions, a process crucial for the proper functioning of cells and organisms. Disruptions in sodium balance can lead to various health problems.
-
Industrial Processes: Sodium compounds, derived from Na⁺, are widely used in various industrial processes, including the production of glass, soap, and paper. Understanding the chemistry of Na⁺ is essential for optimizing these processes and minimizing environmental impact.
Advanced Concepts Related to Na⁺
While the Lewis dot diagram provides a basic representation of Na⁺, a deeper understanding requires considering advanced concepts:
-
Quantum Mechanics: The accurate description of the electronic structure of Na⁺ requires quantum mechanical calculations that go beyond the simple visual representation of Lewis dots. Quantum mechanics provides a more precise understanding of electron orbitals and energy levels.
-
Crystal Structure: In ionic solids like NaCl, the arrangement of Na⁺ and Cl⁻ ions follows a specific crystal structure. Understanding this structure helps explain the macroscopic properties of the solid, such as its melting point and hardness.
-
Spectroscopy: Spectroscopic techniques can provide detailed information about the electronic energy levels of Na⁺. This information is crucial for understanding the interactions of Na⁺ with light and other forms of electromagnetic radiation.
Conclusion
The Lewis dot diagram for Na⁺, while seemingly simple, is a powerful tool for understanding the fundamental principles of ionic bonding, the octet rule, and the reactivity of elements. Its simplicity allows for a clear visual representation of the valence electron configuration, which is crucial for predicting chemical behavior. While advanced concepts are needed for a more complete description, the Lewis dot diagram remains an indispensable starting point for studying the chemical behavior of ions like Na⁺ and its role in various chemical and biological processes. Its application extends far beyond the basic level of understanding to encompass complex areas like electrochemistry, material science, and biology. Understanding the Lewis dot structure of Na⁺ forms a bedrock for more advanced concepts within chemistry and related fields.
Latest Posts
Latest Posts
-
Orange Juice Is Acid Or Base
Apr 02, 2025
-
When Does Segregation Occur In Meiosis
Apr 02, 2025
-
A Worm Is Living Inside A Cow
Apr 02, 2025
-
What Plane Divides The Body Into Front And Back Portions
Apr 02, 2025
-
Who Wrote The Opera The Magic Flute
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Diagram For 2 Individual Ions For Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
