Lewis Dot Diagram For Ionic Bonds
Muz Play
Mar 20, 2025 · 6 min read
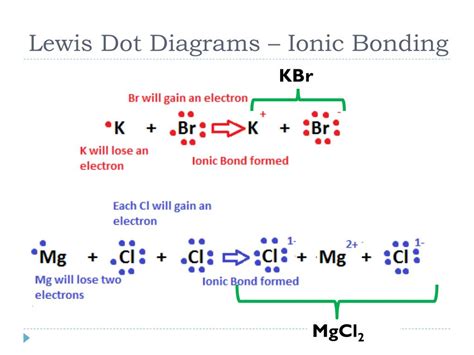
Table of Contents
Lewis Dot Diagrams for Ionic Bonds: A Comprehensive Guide
Lewis dot diagrams, also known as Lewis structures or electron dot diagrams, are simplified representations of the valence electrons in an atom or molecule. These diagrams are invaluable tools for understanding chemical bonding, particularly ionic bonds. This comprehensive guide will delve into the intricacies of using Lewis dot diagrams to illustrate ionic bonding, covering everything from basic principles to complex examples. We'll explore how to draw these diagrams, interpret them, and understand the underlying principles that govern ionic interactions.
Understanding Ionic Bonds
Before diving into Lewis dot diagrams, let's solidify our understanding of ionic bonds. Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom donates one or more electrons to another atom, creating a positively charged cation and a negatively charged anion. The driving force behind this electron transfer is the attainment of a stable electron configuration, often resembling the noble gases (Group 18 elements) with their filled valence shells.
Key Characteristics of Ionic Bonds:
- Electrostatic Attraction: The fundamental force holding ionic compounds together is the strong attraction between positive and negative charges.
- Electron Transfer: Electrons are completely transferred from one atom to another, not shared as in covalent bonds.
- High Melting and Boiling Points: Ionic compounds generally have high melting and boiling points due to the strong electrostatic forces between ions.
- Crystalline Structure: Ionic compounds typically form crystalline structures, arranged in a regular, three-dimensional lattice.
- Solubility in Polar Solvents: Many ionic compounds are soluble in polar solvents like water, where the polar solvent molecules can effectively surround and separate the ions.
Constructing Lewis Dot Diagrams for Ionic Bonds
The beauty of Lewis dot diagrams lies in their simplicity. They provide a visual representation of the valence electrons, making it easier to understand electron transfer during ionic bond formation. Here's a step-by-step guide:
1. Determine the Valence Electrons:
The first crucial step is identifying the number of valence electrons for each atom involved in the ionic bond. Valence electrons are the electrons in the outermost shell of an atom, and they are the ones participating in chemical bonding. The number of valence electrons can be easily determined by looking at the group number (columns) of the element in the periodic table. For example:
- Group 1 (Alkali Metals): 1 valence electron (e.g., Li, Na, K)
- Group 2 (Alkaline Earth Metals): 2 valence electrons (e.g., Be, Mg, Ca)
- Group 17 (Halogens): 7 valence electrons (e.g., F, Cl, Br)
- Group 18 (Noble Gases): 8 valence electrons (except for Helium with 2) (e.g., Ne, Ar, Kr)
2. Represent Valence Electrons with Dots:
Each valence electron is represented by a dot placed around the elemental symbol. It's customary to place one dot on each side of the symbol before pairing them up. This ensures an even distribution of electrons around the atom. For example, oxygen (Group 16, 6 valence electrons) would be represented as:
..
:O:
..
3. Illustrate Electron Transfer:
For ionic bonds, the next step involves showing the transfer of electrons from the metal (cation) to the non-metal (anion). The metal atom will lose electrons to achieve a stable octet (or duet for hydrogen), while the non-metal atom will gain electrons to achieve a stable octet. Arrows can be used to represent the direction of electron transfer.
4. Show the Formation of Ions:
After the electron transfer, the metal atom becomes a positively charged cation (indicated by a "+" superscript), while the non-metal atom becomes a negatively charged anion (indicated by a "-" superscript). The resulting Lewis dot diagrams will show the cation with no dots (if it loses all valence electrons) and the anion with a complete octet.
Examples of Lewis Dot Diagrams for Ionic Bonds
Let's illustrate the process with a few examples:
Example 1: Sodium Chloride (NaCl)
Sodium (Na) is in Group 1, having 1 valence electron. Chlorine (Cl) is in Group 17, having 7 valence electrons.
- Sodium: Na•
- Chlorine: :Cl•
Sodium donates its single valence electron to chlorine:
Na• + :Cl• → Na⁺ + :Cl:⁻
The resulting ionic compound, NaCl, is held together by the electrostatic attraction between the Na⁺ cation and the Cl⁻ anion.
Example 2: Magnesium Oxide (MgO)
Magnesium (Mg) is in Group 2, having 2 valence electrons. Oxygen (O) is in Group 16, having 6 valence electrons.
- Magnesium: Mg••
- Oxygen: :O••
Magnesium donates two electrons to oxygen:
Mg•• + :O•• → Mg²⁺ + :Ö:²⁻
The resulting ionic compound, MgO, is held together by the electrostatic attraction between the Mg²⁺ cation and the O²⁻ anion.
Example 3: Aluminum Oxide (Al₂O₃)
This example demonstrates a slightly more complex scenario involving multiple ions. Aluminum (Al) is in Group 13, having 3 valence electrons, while oxygen (O) is in Group 16, having 6 valence electrons. To achieve charge neutrality, two aluminum atoms will donate a total of six electrons to three oxygen atoms.
- Aluminum: Al•••
- Oxygen: :O••
2Al••• + 3:O•• → 2Al³⁺ + 3:Ö:²⁻
Limitations of Lewis Dot Diagrams
While incredibly helpful, Lewis dot diagrams have some limitations:
- Simple Representation: They are a simplified model and don't accurately represent the complexities of electron distribution in real molecules.
- Ignores 3D Structure: They don't depict the three-dimensional arrangement of atoms within a molecule or crystal lattice.
- Limited Applicability to Complex Molecules: They become less practical and more difficult to interpret for larger, more complex molecules.
- Doesn't Show Bond Polarity: While useful for ionic bonds, they don't explicitly show the polarity of bonds in covalent molecules.
Advanced Applications and Considerations
Lewis dot diagrams can be expanded upon to address more nuanced aspects of chemical bonding. Concepts like formal charge and resonance structures can provide a more complete picture of electron distribution and molecular stability.
Formal Charge: This concept helps determine the most plausible Lewis structure among multiple possibilities by assigning charges to individual atoms based on electron distribution.
Resonance Structures: For certain molecules, multiple valid Lewis structures can be drawn. Resonance structures represent the average distribution of electrons, implying that the actual structure is a hybrid of these different possibilities.
Conclusion
Lewis dot diagrams provide a fundamental yet powerful tool for visualizing ionic bonds and understanding the electron transfer process that underlies their formation. While simplified, they offer a clear and accessible method to grasp the core concepts of ionic bonding and serve as a stepping stone for understanding more complex aspects of chemical bonding. By mastering the construction and interpretation of Lewis dot diagrams, students can build a strong foundation in chemistry and effectively approach more advanced topics. Remember, practice is key to mastering this valuable skill. Work through numerous examples, and you will soon find yourself confidently depicting the electron interactions that govern the world of ionic compounds.
Latest Posts
Latest Posts
-
The Mean Of The Sample Means
Mar 21, 2025
-
Is Sulfur More Electronegative Than Oxygen
Mar 21, 2025
-
Color The Neuron And Neuroglial Cells Answer Key
Mar 21, 2025
-
The Basic Unit Of Life Is The
Mar 21, 2025
-
Calorimetry And Hesss Law Pre Lab Answers
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Diagram For Ionic Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
