Lewis Dot Structures For Polyatomic Ions
Muz Play
Mar 22, 2025 · 6 min read
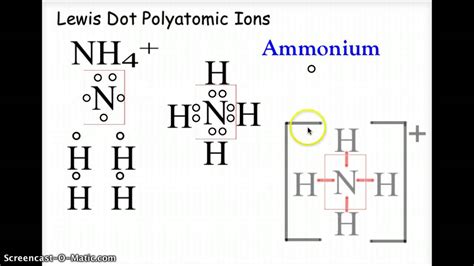
Table of Contents
Lewis Dot Structures for Polyatomic Ions: A Comprehensive Guide
Lewis dot structures are fundamental tools in chemistry used to represent the bonding and arrangement of atoms within molecules and polyatomic ions. Understanding how to draw these structures is crucial for predicting molecular geometry, polarity, and reactivity. This comprehensive guide delves into the intricacies of constructing Lewis dot structures specifically for polyatomic ions, offering a step-by-step approach and numerous examples to solidify your understanding.
What are Polyatomic Ions?
Before diving into the construction of Lewis structures, let's clarify what polyatomic ions are. A polyatomic ion is a charged chemical species composed of two or more atoms covalently bonded together. Unlike monatomic ions (like Na⁺ or Cl⁻) which consist of a single charged atom, polyatomic ions retain their atomic structure even while carrying an overall charge. Examples include:
- Nitrate ion (NO₃⁻): A crucial component in fertilizers and explosives.
- Sulfate ion (SO₄²⁻): Found in many minerals and acids.
- Ammonium ion (NH₄⁺): A common cation in many salts.
- Phosphate ion (PO₄³⁻): Essential for biological processes, including energy transfer.
- Hydroxide ion (OH⁻): A common base in aqueous solutions.
Steps to Draw Lewis Dot Structures for Polyatomic Ions
Constructing Lewis structures for polyatomic ions follows a slightly modified process compared to neutral molecules. Here's a step-by-step guide:
Step 1: Calculate the Total Number of Valence Electrons
This step is critical. Remember to account for the charge of the ion. For anions (negatively charged ions), add the absolute value of the charge to the total number of valence electrons. For cations (positively charged ions), subtract the absolute value of the charge.
Example: For the sulfate ion (SO₄²⁻):
- Sulfur (S) has 6 valence electrons.
- Oxygen (O) has 6 valence electrons each, and there are four oxygen atoms, contributing a total of 24 valence electrons.
- The 2- charge adds 2 more electrons.
Total valence electrons = 6 + 24 + 2 = 32 electrons
Step 2: Identify the Central Atom
Usually, the least electronegative atom (excluding hydrogen) serves as the central atom. However, there are exceptions. In some cases, you might need to consider the atom's capacity to form bonds. For instance, carbon often acts as a central atom due to its ability to form four bonds.
Example: In SO₄²⁻, sulfur is the central atom.
Step 3: Arrange the Atoms and Connect Them with Single Bonds
Place the central atom in the center and arrange the other atoms around it. Connect each surrounding atom to the central atom using a single bond (represented by a line). This uses up a pair of electrons for each bond.
Example: In SO₄²⁻, connect each oxygen atom to the central sulfur atom with a single bond. This uses 8 electrons (4 bonds × 2 electrons/bond).
Step 4: Distribute the Remaining Electrons as Lone Pairs
After forming the single bonds, distribute the remaining valence electrons as lone pairs (two electrons represented by a pair of dots) to the surrounding atoms, starting with the most electronegative atoms, until each atom (except hydrogen) has an octet (eight electrons).
Example: In SO₄²⁻, after forming the four single bonds, we have 32 - 8 = 24 electrons remaining. Distribute these as lone pairs around the oxygen atoms. Each oxygen atom will receive three lone pairs (6 electrons), using up all 24 remaining electrons.
Step 5: Check for Octet Rule Satisfaction
Confirm that all atoms (except hydrogen, which follows the duet rule with two electrons) satisfy the octet rule. If any atoms lack an octet, you'll need to form multiple bonds (double or triple bonds).
Example: In our SO₄²⁻ example, all oxygen atoms have an octet, and the sulfur atom has more than an octet (12 electrons). This is acceptable for elements in the third period and beyond.
Step 6: Formal Charge Calculation (Optional but Recommended)
Calculating formal charges helps determine the most stable Lewis structure, especially when resonance structures are possible. The formal charge is calculated as:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
A lower formal charge on each atom generally indicates a more stable structure.
Step 7: Consider Resonance Structures (if applicable)
If multiple valid Lewis structures can be drawn for a polyatomic ion, they are called resonance structures. The actual structure is a hybrid of these resonance forms. Use double-headed arrows to indicate resonance.
Examples of Lewis Dot Structures for Polyatomic Ions
Let's walk through a few more examples:
Example 1: Nitrate Ion (NO₃⁻)
- Valence Electrons: 5 (N) + 3 * 6 (O) + 1 (charge) = 24 electrons
- Central Atom: Nitrogen (N)
- Single Bonds: Connect each oxygen atom to nitrogen with a single bond (6 electrons used).
- Lone Pairs: Distribute the remaining 18 electrons (24 - 6 = 18) as lone pairs around the oxygen atoms.
- Octet Check: Nitrogen only has 6 electrons. To satisfy the octet rule, one of the oxygen atoms forms a double bond with nitrogen. This leads to resonance structures.
Example 2: Ammonium Ion (NH₄⁺)
- Valence Electrons: 5 (N) + 4 * 1 (H) - 1 (charge) = 8 electrons
- Central Atom: Nitrogen (N)
- Single Bonds: Connect each hydrogen atom to nitrogen with a single bond (8 electrons used).
- Lone Pairs: No lone pairs are needed, as all electrons are involved in bonding.
- Octet Check: Nitrogen has an octet, and each hydrogen has a duet.
Example 3: Phosphate Ion (PO₄³⁻)
- Valence Electrons: 5 (P) + 4 * 6 (O) + 3 (charge) = 32 electrons
- Central Atom: Phosphorus (P)
- Single Bonds: Connect each oxygen atom to phosphorus with a single bond (8 electrons used).
- Lone Pairs: Distribute the remaining 24 electrons (32 - 8 = 24) as lone pairs around the oxygen atoms.
- Octet Check: All atoms satisfy the octet rule.
Beyond the Basics: Exceptions and Advanced Concepts
While the octet rule is a helpful guideline, there are exceptions:
- Incomplete Octet: Some atoms, particularly boron, can have fewer than eight electrons in their valence shell.
- Expanded Octet: Elements in the third period and beyond can accommodate more than eight electrons in their valence shell, as seen in SO₄²⁻.
- Odd-Electron Species: Some molecules or ions have an odd number of valence electrons and cannot satisfy the octet rule for all atoms.
Mastering Lewis structures is a cornerstone of understanding chemical bonding. By systematically following the steps outlined above and practicing with numerous examples, you'll gain confidence in predicting the structure and properties of polyatomic ions. Remember that while formal charges and resonance structures add complexity, they are valuable tools for understanding the nuances of chemical bonding and predicting molecular behavior. Continuous practice is key to developing proficiency in this essential skill.
Latest Posts
Latest Posts
-
Where In The Cell Does Fermentation Occur
Mar 23, 2025
-
What Is The Acceptable Macronutrient Distribution Range For Carbohydrates
Mar 23, 2025
-
How To Show Vectors Are Linearly Independent
Mar 23, 2025
-
An Equation Stating 2 Ratios Are Equal
Mar 23, 2025
-
During Glycolysis Glucose Is Broken Down Into Two Molecules Of
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structures For Polyatomic Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
