Lithium Aluminum Hydride Reduction Of Carboxylic Acids
Muz Play
Mar 24, 2025 · 6 min read
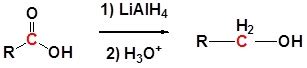
Table of Contents
Lithium Aluminum Hydride Reduction of Carboxylic Acids: A Comprehensive Guide
The reduction of carboxylic acids to primary alcohols is a fundamental transformation in organic chemistry, crucial for the synthesis of a wide variety of molecules. While several reducing agents can achieve this, lithium aluminum hydride (LiAlH₄), a powerful hydride reducing agent, stands out for its effectiveness and broad applicability. This article delves deep into the mechanism, applications, limitations, and safety considerations associated with LiAlH₄ reduction of carboxylic acids.
Understanding Lithium Aluminum Hydride (LiAlH₄)
Lithium aluminum hydride is a complex metal hydride composed of an aluminum atom coordinated to four hydride ions (H⁻) and a lithium cation (Li⁺). This arrangement gives LiAlH₄ its exceptional reducing power. The hydride ions are highly nucleophilic and readily donate their electrons to electrophilic centers, initiating the reduction process. Its strength lies in its ability to reduce a wide range of functional groups, including esters, ketones, aldehydes, and notably, carboxylic acids. However, its reactivity necessitates careful handling and specific reaction conditions.
The Mechanism of Carboxylic Acid Reduction with LiAlH₄
The reduction of a carboxylic acid using LiAlH₄ proceeds through a series of steps. Let's break down the mechanism:
Step 1: Nucleophilic Attack: The hydride ion (H⁻) from LiAlH₄ acts as a nucleophile, attacking the electrophilic carbonyl carbon of the carboxylic acid. This forms an alkoxide intermediate.
Step 2: Formation of a Tetrahedral Intermediate: The addition of the hydride ion to the carbonyl carbon creates a tetrahedral intermediate. This intermediate is unstable and quickly undergoes further reactions.
Step 3: Elimination of Aluminate: The alkoxide intermediate is further attacked by another hydride ion from LiAlH₄. This leads to the elimination of an aluminate ion (AlH₃O⁻). This aluminate byproduct is relatively stable and doesn't interfere with the subsequent steps.
Step 4: Protonation: The final step involves the protonation of the resulting alkoxide ion. This is typically achieved using a weak acid like water or dilute mineral acid during the workup process. This protonation yields the primary alcohol as the final product.
Practical Considerations for LiAlH₄ Reduction
Successful LiAlH₄ reduction of carboxylic acids requires careful attention to several factors:
Solvent Selection:
The choice of solvent plays a significant role. Diethyl ether and tetrahydrofuran (THF) are commonly employed solvents due to their ability to dissolve both LiAlH₄ and the carboxylic acid substrate. These solvents are relatively inert under the reaction conditions and facilitate the reaction progress.
Reaction Temperature and Time:
The reaction is usually carried out under anhydrous conditions, typically at reflux (boiling point of the solvent). The reaction time varies depending on the substrate, but often ranges from several hours to overnight. Controlling the temperature is essential to prevent side reactions and improve yield.
Stoichiometry:
Generally, a slight excess of LiAlH₄ (typically 1.2-1.5 equivalents) is used to ensure complete reduction. This excess accounts for potential side reactions or incomplete reduction.
Workup Procedure:
The workup procedure is critical to isolate the primary alcohol product. Carefully controlled addition of water or dilute acid (like sulfuric acid) is crucial. This step decomposes the excess LiAlH₄ and protonates the alkoxide intermediate, yielding the primary alcohol. The organic layer is then separated, washed, and dried. Finally, the solvent is removed under reduced pressure to isolate the desired product. This procedure must be carried out cautiously to avoid exothermic reactions and potential hazards.
Purification:
Purification of the final product often involves techniques like distillation, recrystallization, or chromatography. The method of choice depends on the properties of the product and the presence of any impurities.
Applications of LiAlH₄ Reduction of Carboxylic Acids
The reduction of carboxylic acids to primary alcohols using LiAlH₄ has numerous applications in various areas of organic synthesis:
-
Pharmaceutical Industry: The synthesis of numerous pharmaceutical drugs relies on this reduction. Many active pharmaceutical ingredients (APIs) contain alcohol functional groups derived from the reduction of carboxylic acids.
-
Natural Product Synthesis: The synthesis of many natural products requires the reduction of carboxylic acids to obtain the necessary alcohol building blocks.
-
Materials Science: This transformation plays a role in the synthesis of various polymeric materials, where alcohols serve as crucial intermediates or components.
-
Organic Chemistry Research: As a powerful and versatile reducing agent, LiAlH₄ is indispensable in academic research for the synthesis of novel compounds and the exploration of chemical transformations.
Limitations of LiAlH₄ Reduction
Despite its advantages, LiAlH₄ reduction has some limitations:
-
Sensitivity to Moisture and Air: LiAlH₄ is highly reactive with water and air, making rigorous anhydrous conditions essential. Exposure to moisture leads to rapid decomposition, generating hydrogen gas which poses a safety risk.
-
Reactivity with other Functional Groups: LiAlH₄ can reduce other functional groups besides carboxylic acids, including esters, ketones, and aldehydes. This can complicate the reaction if multiple functional groups are present in the substrate. Selective reduction requires careful planning and modification of reaction conditions.
-
Toxicity: LiAlH₄ is a highly toxic substance that requires careful handling. Appropriate safety measures, including gloves and personal protective equipment, are essential to prevent exposure.
Safety Precautions
Handling LiAlH₄ demands strict adherence to safety protocols due to its pyrophoric nature and reactivity with water.
-
Anhydrous Conditions: All glassware and reagents must be thoroughly dried. The reaction should be performed under an inert atmosphere (e.g., nitrogen or argon).
-
Personal Protective Equipment: Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat. Work in a well-ventilated area or under a fume hood.
-
Slow Addition: LiAlH₄ should be added slowly and cautiously to the reaction mixture to control the reaction exothermicity and prevent excessive heat generation.
-
Careful Quenching: The reaction must be carefully quenched with water or dilute acid. This step should be performed slowly and with proper cooling to prevent vigorous reaction and potential hazards.
-
Waste Disposal: Dispose of all waste materials according to appropriate safety regulations.
Alternatives to LiAlH₄ Reduction
While LiAlH₄ is a powerful reducing agent, alternative reagents can be considered for certain substrates or when specific reaction conditions are needed. These alternatives often offer higher selectivity or milder reaction conditions:
-
Diborane (B₂H₆): Diborane is a milder reducing agent and is less reactive than LiAlH₄. It can selectively reduce carboxylic acids in the presence of other reducible functional groups.
-
Boron Trifluoride Etherate (BF₃·OEt₂): This Lewis acid can facilitate the reduction of carboxylic acids under different conditions.
-
Sodium Borohydride (NaBH₄): Though less powerful than LiAlH₄, NaBH₄ can reduce some activated carboxylic acids, but usually requires harsh reaction conditions or activation via other reagents.
Conclusion
The LiAlH₄ reduction of carboxylic acids remains a highly valuable and widely used method for converting carboxylic acids into primary alcohols. Its effectiveness and versatility make it a cornerstone of organic synthesis. However, the inherent reactivity and toxicity of LiAlH₄ necessitate careful planning, meticulous execution, and strict adherence to safety protocols. Understanding the mechanism, reaction parameters, and potential limitations is crucial for successful synthesis and safe handling of this potent reducing agent. Careful consideration of alternative reducing agents may be appropriate in cases where the limitations of LiAlH₄ present challenges. The choice of reagent should always be tailored to the specific needs of the synthesis and the nature of the substrate.
Latest Posts
Latest Posts
-
Comparison Test For Convergence And Divergence
Mar 26, 2025
-
What Is Pathophysiology Of A Disease
Mar 26, 2025
-
Chi Square Calculator For Goodness Of Fit
Mar 26, 2025
-
Advertising Goals Listed In An Advertising Plan Must Be
Mar 26, 2025
-
What Do Hypotheses Theories And Laws Have In Common
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Lithium Aluminum Hydride Reduction Of Carboxylic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
