Lock And Key Model Of Enzyme Action
Muz Play
Mar 19, 2025 · 6 min read
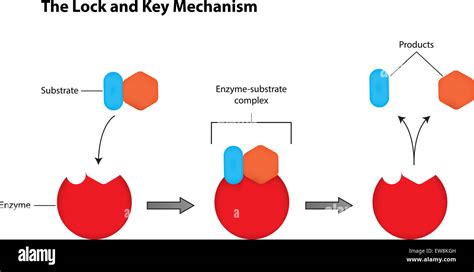
Table of Contents
The Lock and Key Model of Enzyme Action: A Comprehensive Guide
The lock and key model, a cornerstone of biochemistry, elegantly explains the remarkable specificity and efficiency of enzyme-substrate interactions. While modern understanding has evolved beyond this initial model to incorporate the induced-fit model, understanding the lock and key mechanism remains crucial for grasping the fundamentals of enzyme catalysis. This comprehensive guide delves deep into the intricacies of the lock and key model, exploring its strengths, limitations, and relevance in the broader context of enzyme function.
Understanding the Basics: Enzymes and Substrates
Before diving into the model itself, let's establish a foundational understanding of enzymes and substrates. Enzymes are biological catalysts, predominantly proteins, that significantly accelerate the rate of biochemical reactions within living organisms. They achieve this remarkable feat without being consumed in the process. Substrates are the molecules upon which enzymes act, undergoing transformations to form products.
The specificity of enzyme action is astounding. Each enzyme typically interacts with only one or a very limited range of substrates, showcasing an exquisite selectivity. This specificity is the key to the highly organized and regulated metabolic processes within cells. The lock and key model provides a framework for understanding this specificity.
The Lock and Key Analogy: A Simple Explanation
The lock and key model likens the enzyme to a lock and the substrate to a key. Only the correctly shaped "key" (substrate) can fit into the "lock" (enzyme's active site), initiating the catalytic process. The active site is a specific three-dimensional region within the enzyme's structure, possessing a unique shape and chemical environment perfectly suited to bind the substrate.
Imagine a perfectly crafted key, designed to fit only one specific lock. This is analogous to the enzyme-substrate interaction. The substrate's shape complements the active site's shape with remarkable precision. This precise fit is crucial for effective binding and subsequent catalysis.
Key features of the lock and key model:
- Specificity: Only the correct substrate can bind to the enzyme's active site.
- Complementary shapes: The substrate's shape precisely matches the active site's shape.
- Binding: The substrate binds to the active site through various non-covalent interactions, such as hydrogen bonds, van der Waals forces, and hydrophobic interactions.
- Catalysis: Once bound, the enzyme facilitates the reaction, converting the substrate into products.
- Release: After the reaction, the products are released, leaving the enzyme free to catalyze another reaction.
The Role of the Active Site: A Closer Look
The active site is not merely a passive receptacle for the substrate. Its intricate structure plays a pivotal role in the catalytic process. The active site's amino acid residues are arranged in a specific three-dimensional configuration, contributing to the following crucial functions:
- Substrate Binding: Precisely positioned amino acid side chains form non-covalent interactions with the substrate, ensuring a strong and specific binding.
- Orientation: The active site holds the substrate in the optimal orientation for the reaction to occur. This proper alignment maximizes the effectiveness of the catalytic process.
- Catalysis: Amino acid residues within the active site directly participate in the catalytic mechanism, often through acid-base catalysis, covalent catalysis, or metal ion catalysis. These mechanisms lower the activation energy of the reaction, dramatically speeding up its rate.
Strengths of the Lock and Key Model
The lock and key model, despite its limitations, offers several significant advantages:
- Simplicity: It provides a simple and intuitive explanation of enzyme specificity and substrate binding. This ease of understanding makes it an excellent introductory model for students learning about enzyme function.
- Explanatory Power: It successfully explains the remarkable selectivity observed in many enzyme-catalyzed reactions. The strict complementarity between enzyme and substrate dictates that only specific substrates can undergo catalysis.
- Foundation for further models: While not a completely accurate representation of all enzyme-substrate interactions, it serves as a foundational model upon which more sophisticated models, like the induced-fit model, are built.
Limitations of the Lock and Key Model
While elegant and conceptually straightforward, the lock and key model does have limitations:
- Rigidity: It assumes a rigid structure for both the enzyme and the substrate. This contradicts experimental evidence demonstrating conformational changes in both molecules during the catalytic process.
- Lack of Dynamic Interaction: It doesn't fully capture the dynamic nature of enzyme-substrate interactions. The model doesn't account for the induced conformational changes that often occur upon substrate binding.
- Inability to Explain Enzyme Regulation: It fails to explain mechanisms of enzyme regulation, such as allosteric regulation or feedback inhibition, which involve conformational changes affecting enzyme activity.
- Oversimplification: The model simplifies the complexity of enzyme active sites, which are often composed of multiple binding pockets and interact with substrates through a multitude of non-covalent forces.
The Induced-Fit Model: An Enhancement
The limitations of the lock and key model led to the development of the induced-fit model, a more refined and accurate representation of enzyme-substrate interactions. This model proposes that the enzyme's active site is flexible and undergoes conformational changes upon substrate binding.
The substrate's binding induces a conformational change in the enzyme, creating a more complementary shape for optimal binding and catalysis. This dynamic interaction ensures a tighter fit, enhancing the catalytic efficiency and specificity of the enzyme. The induced-fit model effectively addresses many of the shortcomings of the lock and key model.
Examples of Lock and Key Mechanism in Action
Although the induced-fit model is currently favored, understanding the lock and key mechanism is still valuable as it helps to visualize enzyme specificity. Several examples demonstrate how enzymes exhibit substrate selectivity, underpinning the fundamental concepts of the lock and key model:
- Hexokinase: This enzyme phosphorylates hexoses, showing preference for glucose. The active site's shape and chemical environment favor glucose over other hexoses, illustrating substrate specificity.
- Chymotrypsin: This protease cleaves peptide bonds selectively after bulky hydrophobic amino acid residues. The active site's hydrophobic pocket accommodates these residues, leading to specific cleavage patterns.
- Lysozyme: This enzyme breaks down bacterial cell walls. Its active site specifically binds to the polysaccharide chains in bacterial cell walls, enabling efficient hydrolysis. The complementarity between the active site and the substrate is crucial for its antibacterial activity.
Conclusion: The Enduring Relevance of the Lock and Key Model
While the induced-fit model provides a more comprehensive and accurate description of enzyme-substrate interactions, the lock and key model remains a valuable educational tool. Its simplicity and intuitive nature allow for a basic understanding of enzyme specificity and catalysis. Furthermore, it serves as a stepping stone to more advanced models, facilitating a progressive understanding of enzyme function. The lock and key model's enduring relevance lies in its ability to provide a foundational framework for comprehending the complex world of enzyme catalysis. It highlights the importance of shape complementarity in achieving enzyme specificity and efficiency, providing a crucial starting point for appreciating the intricate dance between enzymes and substrates. Even with its limitations, its contribution to our understanding of biochemistry remains significant and continues to serve as a cornerstone in biochemical education.
Latest Posts
Latest Posts
-
Where Does Fermentation Occur In A Cell
Mar 19, 2025
-
Work In A N Electric Field
Mar 19, 2025
-
Introduction To Acids And Bases Worksheet
Mar 19, 2025
-
What Color Is The Pancreas In A Frog
Mar 19, 2025
-
How To Find The Matrix Of A Linear Transformation
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Lock And Key Model Of Enzyme Action . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
