Matter Is Neither Created Or Destroyed
Muz Play
Mar 29, 2025 · 6 min read
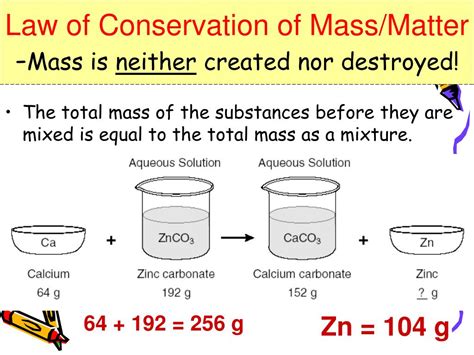
Table of Contents
Matter: Neither Created Nor Destroyed – A Deep Dive into the Law of Conservation of Mass
The universe, in all its breathtaking complexity, abides by fundamental laws. One of the most crucial and enduring of these is the law of conservation of mass, which states that matter can neither be created nor destroyed, only transformed. This seemingly simple principle underpins countless scientific disciplines and has profound implications for our understanding of the cosmos. This article delves deep into this cornerstone of physics and chemistry, exploring its historical development, scientific basis, applications, and the nuances and exceptions that add layers of intrigue to this fundamental law.
A Historical Perspective: From Alchemy to Modern Physics
The concept of matter's immutability has ancient roots, entangled with the alchemical pursuits of transforming base metals into gold. While their methods were ultimately flawed, alchemists inadvertently laid the groundwork for future discoveries by meticulously documenting their experiments and observations. The true scientific understanding of mass conservation emerged much later.
Antoine Lavoisier, considered the "father of modern chemistry," played a pivotal role in establishing the law in the late 18th century. Through meticulous experiments, he demonstrated that during chemical reactions, the total mass of the reactants always equaled the total mass of the products. This crucial observation shattered the prevailing Aristotelian notion of elements transforming into each other, establishing the groundwork for a quantitative understanding of chemical processes. Lavoisier's work cemented the law of conservation of mass, albeit within the limited understanding of matter available at that time.
The Role of Precision Measurement
Lavoisier's success hinged upon precise measurement. He meticulously weighed reactants and products, demonstrating that mass remained constant even though the substances had changed form. This emphasis on quantitative analysis became a cornerstone of scientific methodology, highlighting the importance of rigorous experimentation in validating scientific laws. The development of increasingly sophisticated measuring instruments over time has further strengthened our understanding and ability to verify this fundamental principle.
The Scientific Basis: Atoms and Molecules
The microscopic world of atoms and molecules provides a deeper understanding of why matter is conserved. Chemical reactions involve the rearrangement of atoms within molecules. Atoms, the fundamental building blocks of matter, are neither created nor destroyed during ordinary chemical changes. They simply bond and unbond in different configurations, leading to the formation of new substances with different properties.
Breaking Bonds, Forming New Ones
Imagine a simple chemical reaction like the burning of wood. Wood, primarily composed of cellulose and lignin, reacts with oxygen in the air. The chemical bonds within the wood and oxygen molecules break, and the atoms rearrange to form carbon dioxide, water, and ash. While the substances have transformed drastically, the total number of atoms of each element (carbon, hydrogen, oxygen) remains the same. The mass, therefore, remains constant.
Beyond Chemical Reactions: Nuclear Reactions and Einstein's E=mc²
The law of conservation of mass, as originally stated, holds true for most chemical reactions. However, the advent of nuclear physics revealed a more nuanced picture. Nuclear reactions, involving changes within the atom's nucleus, can lead to a slight change in mass.
Einstein's famous equation, E=mc², demonstrated the equivalence of energy and mass. This equation reveals that a small amount of mass can be converted into a tremendous amount of energy, and vice-versa. In nuclear reactions, such as fission and fusion, a small fraction of the mass is converted into energy, seemingly violating the classical law of conservation of mass.
The Law of Conservation of Mass-Energy
This apparent discrepancy led to the formulation of the law of conservation of mass-energy. This broader law states that the total amount of mass and energy in a closed system remains constant. While mass can be converted to energy (and vice-versa), the total quantity of mass-energy remains unchanged. This revised law encompasses both chemical and nuclear reactions, providing a more complete and accurate representation of the principle.
Applications Across Disciplines
The principle of mass conservation has far-reaching implications across various scientific fields:
Chemistry: Stoichiometry and Chemical Calculations
In chemistry, the law is fundamental to stoichiometry, the quantitative study of reactants and products in chemical reactions. It allows chemists to accurately predict the amounts of substances involved in a reaction, crucial for designing and optimizing chemical processes in various industries, from pharmaceuticals to manufacturing.
Environmental Science: Pollution Control and Resource Management
Understanding mass conservation is crucial for environmental science. It helps in tracking pollutants and their transformations in the environment, enabling the development of effective pollution control strategies. Furthermore, it is vital for resource management, allowing for accurate assessment of resource availability and waste generation.
Geology: Understanding Earth's Processes
The law plays a significant role in geological studies. It helps in understanding the formation and transformation of rocks and minerals, volcanic eruptions, and other geological processes. The distribution of elements in the Earth's crust is a direct consequence of this fundamental law.
Astrophysics: Stellar Evolution and Nucleosynthesis
In astrophysics, the law of conservation of mass-energy is essential for understanding stellar evolution. Stars generate energy through nuclear fusion, converting mass into energy. The processes of nucleosynthesis, where heavier elements are formed from lighter ones within stars, are governed by this principle. It helps us understand the origin of elements in the universe.
Exceptions and Nuances
While the law of conservation of mass-energy holds true for most situations, there are a few exceptions and nuances worth considering:
Quantum Mechanics: Particle-Antiparticle Annihilation
In the realm of quantum mechanics, certain phenomena seemingly challenge the law. For example, particle-antiparticle annihilation involves the complete conversion of mass into energy when a particle and its antiparticle collide. However, even in this case, the total mass-energy remains conserved. The apparent "disappearance" of mass is accounted for by the release of equivalent energy.
Extremely High Energies: Relativistic Effects
At extremely high energies, close to the speed of light, relativistic effects become significant. The mass of an object increases with its velocity, as described by Einstein's theory of special relativity. While mass is not strictly conserved in this context, the total mass-energy remains constant, reinforcing the more comprehensive law of conservation of mass-energy.
Conclusion: An Enduring Principle
The law of conservation of mass, in its refined form as the law of conservation of mass-energy, stands as a testament to the underlying order and predictability of the universe. From the simplest chemical reactions to the most complex nuclear processes, this fundamental principle provides a framework for understanding the transformations of matter and energy. While nuances and exceptions exist at the extremes of energy scales or within the quantum realm, the core principle remains profoundly influential across countless scientific disciplines. Its enduring validity underscores its importance as a cornerstone of scientific understanding and continues to inspire further exploration and discovery. The ongoing pursuit of knowledge in physics and chemistry continually reinforces and refines our understanding of this essential principle governing the universe.
Latest Posts
Latest Posts
-
Are All Ionic Compounds Strong Electrolytes
Apr 01, 2025
-
How Do You Use A Balance
Apr 01, 2025
-
Nucleotide Excision Repair Vs Mismatch Repair
Apr 01, 2025
-
What Is The Difference Between Religious And Ethnic Groups
Apr 01, 2025
-
What Is Found In Both Dna And Rna
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Matter Is Neither Created Or Destroyed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
