Metallic Character Of Elements In Periodic Table
Muz Play
Mar 21, 2025 · 6 min read
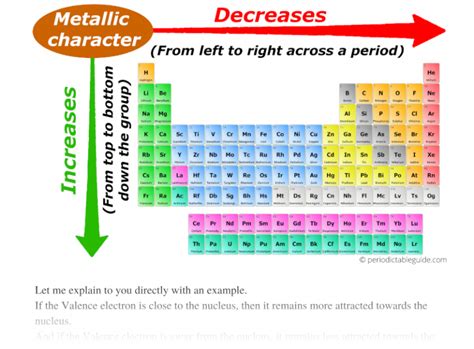
Table of Contents
Metallic Character of Elements in the Periodic Table: A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. One of the key periodic trends is metallic character, which describes the tendency of an element to lose electrons and form positive ions (cations). Understanding metallic character is crucial for predicting chemical behavior and explaining the properties of various materials. This article delves deep into the concept of metallic character, exploring its trends across the periodic table, its relationship to other properties, and its significant implications in various fields.
What is Metallic Character?
Metallic character is a measure of how readily an atom can lose electrons. Elements with high metallic character are easily ionized, readily donating electrons to form stable cations. This ability stems from their relatively low ionization energies and electron affinities. These elements typically exhibit properties characteristic of metals, such as:
- Electrical Conductivity: Free electrons are responsible for the excellent electrical conductivity of metals.
- Thermal Conductivity: Metals efficiently transfer heat due to the mobility of their electrons.
- Malleability and Ductility: The ability to be hammered into sheets (malleability) and drawn into wires (ductility) arises from the sea of delocalized electrons holding metal atoms together in a flexible manner.
- Luster: The shiny appearance of metals is a result of the interaction of light with their delocalized electrons.
Periodic Trends in Metallic Character
Metallic character shows a clear pattern across the periodic table:
1. Across a Period (Left to Right):
As you move from left to right across a period, the metallic character generally decreases. This is because the effective nuclear charge increases while the shielding effect remains relatively constant. The increased nuclear pull holds the valence electrons more tightly, making them less likely to be lost. Atoms become less willing to donate electrons, resulting in decreased metallic character. The elements transition from highly reactive alkali metals to relatively unreactive nonmetals.
2. Down a Group (Top to Bottom):
Moving down a group, the metallic character generally increases. This is primarily due to the increasing atomic size. As you go down a group, the valence electrons are located further from the nucleus, experiencing less effective nuclear charge. This results in a weaker attraction between the nucleus and the valence electrons, making it easier for them to be lost and increasing metallic character. Consequently, elements at the bottom of a group are more metallic than those at the top.
Factors Affecting Metallic Character
Several factors contribute to an element's metallic character:
- Atomic Radius: Larger atomic radius leads to increased metallic character because the valence electrons are farther from the nucleus and are more easily lost.
- Ionization Energy: Lower ionization energy indicates that an element readily loses electrons, thus possessing high metallic character. The easier it is to remove an electron, the more metallic the element.
- Electronegativity: Lower electronegativity signifies a weaker attraction for electrons, leading to higher metallic character. Elements with low electronegativity are more likely to donate electrons than to accept them.
- Effective Nuclear Charge: A lower effective nuclear charge means less pull on the valence electrons, increasing metallic character. Increased shielding from inner electrons reduces the effect of the nucleus on the outer electrons.
- Electron Shielding: Greater shielding of valence electrons by inner electrons reduces the attractive force from the nucleus, leading to increased metallic character.
Relationship with Other Periodic Properties
Metallic character is closely intertwined with other periodic properties:
- Ionization Energy: A strong inverse relationship exists between metallic character and ionization energy. High metallic character implies low ionization energy.
- Electronegativity: Metallic character and electronegativity exhibit a strong inverse relationship. High metallic character correlates with low electronegativity.
- Electron Affinity: While not as directly correlated, elements with high metallic character generally have low electron affinities, meaning they are less likely to gain electrons.
- Reactivity: Highly metallic elements are generally more reactive, readily participating in chemical reactions by losing electrons.
Examples of Metallic and Non-Metallic Elements
Let's examine specific examples to illustrate the concept:
-
Alkali Metals (Group 1): These elements (Li, Na, K, Rb, Cs, Fr) exhibit the highest metallic character in the periodic table. They have very low ionization energies and readily lose one electron to form +1 cations. Their reactivity increases down the group.
-
Alkaline Earth Metals (Group 2): These elements (Be, Mg, Ca, Sr, Ba, Ra) also exhibit high metallic character, although slightly less than alkali metals. They lose two electrons to form +2 cations. Their reactivity also increases down the group.
-
Transition Metals: These elements show a wide range of metallic character. Their properties are often influenced by factors beyond simple ionization energy and electronegativity, such as d-orbital electron configurations and variable oxidation states.
-
Halogens (Group 17): These elements (F, Cl, Br, I, At) exhibit very low metallic character, preferring to gain electrons to form -1 anions. Their reactivity decreases down the group.
-
Noble Gases (Group 18): These elements (He, Ne, Ar, Kr, Xe, Rn) possess virtually no metallic character, being extremely unreactive due to their stable electron configurations.
Applications and Importance of Metallic Character
Understanding metallic character has significant implications across various fields:
-
Material Science: The metallic character of elements is crucial in designing alloys and other materials with specific properties. For example, the addition of alloying elements can alter the strength, ductility, and corrosion resistance of metals.
-
Chemistry: Predicting the reactivity and chemical behavior of elements is reliant on understanding their metallic character. This knowledge helps in designing chemical reactions and predicting the products formed.
-
Electronics: Metals' electrical conductivity, directly related to their metallic character, is essential for various electronic applications, from wiring to semiconductors.
-
Catalysis: The catalytic activity of many transition metals is linked to their variable oxidation states and metallic character, enabling them to participate in redox reactions.
-
Biology: Certain metal ions play vital roles in biological processes. Their metallic character influences their ability to bind to molecules and participate in enzymatic reactions.
Conclusion
Metallic character is a fundamental property that dictates the behavior and applications of elements. Its periodic trends provide a valuable framework for understanding and predicting chemical properties. The interplay between atomic size, ionization energy, electronegativity, and other factors shapes the extent of metallic character across the periodic table. By grasping these concepts, we gain invaluable insight into the diverse world of elements and their practical significance in various scientific and technological domains. Further research into the nuances of metallic character and its relationship to other properties promises to unveil further insights into the intricate world of chemistry and materials science. The ability to predict and manipulate the metallic character of materials remains a key driver of innovation across numerous fields.
Latest Posts
Latest Posts
-
Derivative Of Sin Cos Tan Sec Csc Cot
Mar 21, 2025
-
What Are Disadvantages Of Sexual Reproduction
Mar 21, 2025
-
Can There Be More Than One Loop In A Circuit
Mar 21, 2025
-
Work Is Change In Kinetic Energy
Mar 21, 2025
-
In An Aqueous Solution The Solvent Is
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Metallic Character Of Elements In Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
