Negatively Charged Particle In The Atom
Muz Play
Apr 07, 2025 · 7 min read
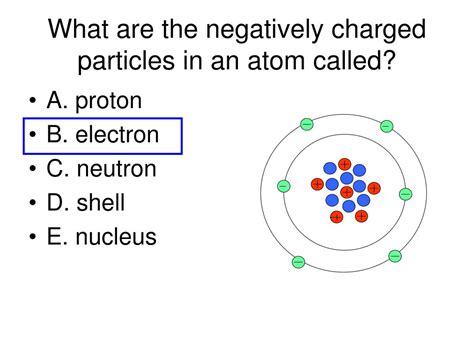
Table of Contents
Negatively Charged Particles in the Atom: A Deep Dive into Electrons
The atom, the fundamental building block of matter, is a fascinating world of subatomic particles. While the overall charge of an atom is typically neutral, its internal structure is a dynamic interplay of positive and negative charges. This article delves into the negatively charged particles within the atom – electrons – exploring their properties, behavior, and significance in various scientific fields. We'll cover their discovery, their role in chemical bonding, their behavior in electric and magnetic fields, and their contribution to our understanding of the universe.
The Discovery of Electrons: A Journey into the Subatomic World
The story of the electron begins with the late 19th and early 20th centuries, a period of groundbreaking discoveries in physics. Before the electron's discovery, the prevailing understanding of the atom was that it was a solid, indivisible entity. However, experiments like cathode ray tube experiments significantly challenged this view.
Cathode Ray Tube Experiments and J.J. Thomson's Contribution
Scientists observed that when a high voltage was applied across a cathode ray tube (a sealed glass tube with electrodes at each end), a beam of particles, called cathode rays, emanated from the cathode (the negatively charged electrode) and traveled to the anode (the positively charged electrode). These rays caused fluorescence when they struck the glass.
J.J. Thomson, through a series of meticulous experiments, demonstrated that these cathode rays were composed of negatively charged particles much smaller than atoms. He measured their charge-to-mass ratio, finding it to be significantly higher than that of any known ion. This groundbreaking discovery, published in 1897, provided the first concrete evidence for the existence of subatomic particles and marked the birth of the understanding of the electron as a fundamental constituent of matter. Thomson's model, often referred to as the "plum pudding model," depicted the atom as a positively charged sphere with negatively charged electrons embedded within it.
Millikan's Oil Drop Experiment: Determining the Electron's Charge
While Thomson determined the charge-to-mass ratio of the electron, it was Robert Millikan's oil drop experiment that precisely measured the elementary charge of the electron. By observing the motion of electrically charged oil droplets in an electric field, Millikan determined the fundamental unit of electric charge, which corresponded to the charge of a single electron. This experiment provided crucial confirmation of Thomson's findings and refined the understanding of the electron's properties.
Properties of Electrons: Mass, Charge, and Spin
Electrons are fundamental particles classified as leptons, meaning they are not composed of smaller constituent particles. Key properties include:
- Charge: Electrons carry a single unit of negative electric charge, conventionally represented as -1. This negative charge is equal in magnitude but opposite in sign to the charge of a proton.
- Mass: Electrons possess a very small mass, approximately 1/1836 the mass of a proton or neutron. This relatively small mass plays a crucial role in their behavior within the atom.
- Spin: Electrons have an intrinsic angular momentum property called spin. This is not a literal spinning motion, but a fundamental quantum mechanical property that affects their magnetic moment. The spin of an electron is quantized, meaning it can only take on specific values, conventionally denoted as +1/2 or -1/2 (often referred to as "spin up" and "spin down").
- Wave-particle duality: Electrons exhibit both wave-like and particle-like properties. This wave-particle duality is a fundamental concept in quantum mechanics, explaining many of the electron's behaviors, such as diffraction and interference patterns observed in electron beams.
Electron Behavior in Atoms: Orbitals and Quantum Numbers
The electrons in an atom do not orbit the nucleus in simple circular paths as initially envisioned. Instead, their behavior is governed by the principles of quantum mechanics. They occupy regions of space called orbitals, which are described by a set of quantum numbers:
- Principal quantum number (n): Determines the energy level of the electron and the size of the orbital. Higher values of n indicate higher energy levels and larger orbitals.
- Azimuthal quantum number (l): Determines the shape of the orbital (s, p, d, f, etc.). For a given n, l can range from 0 to n - 1.
- Magnetic quantum number (ml): Determines the orientation of the orbital in space. For a given l, ml can range from -l to +l.
- Spin quantum number (ms): Specifies the electron's spin, either +1/2 or -1/2.
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This principle dictates the arrangement of electrons within an atom and determines the atom's electron configuration. The electron configuration is crucial in understanding an atom's chemical properties and reactivity.
The Role of Electrons in Chemical Bonding
Electrons are the primary players in chemical bonding, the forces that hold atoms together to form molecules and compounds. The outermost electrons, known as valence electrons, are particularly important in bonding. There are three main types of chemical bonds:
- Ionic bonds: These bonds involve the transfer of electrons from one atom to another. One atom loses electrons (becoming a positively charged ion or cation), and another atom gains electrons (becoming a negatively charged ion or anion). The electrostatic attraction between the oppositely charged ions holds them together.
- Covalent bonds: These bonds involve the sharing of electrons between atoms. Atoms share electrons to achieve a stable electron configuration, typically a full outer electron shell.
- Metallic bonds: These bonds occur in metals, where electrons are delocalized and move freely throughout the metallic lattice. This sea of delocalized electrons accounts for the characteristic properties of metals, such as conductivity and malleability.
Understanding electron configuration and behavior is fundamental to predicting and explaining chemical reactivity and the formation of chemical compounds.
Electron Behavior in Electric and Magnetic Fields
Electrons, being charged particles, interact strongly with electric and magnetic fields.
- Electric fields: An electric field exerts a force on an electron, causing it to accelerate. The direction of the force depends on the direction of the electric field and the sign of the electron's charge.
- Magnetic fields: A magnetic field exerts a force on a moving electron. The force is perpendicular to both the direction of the electron's velocity and the direction of the magnetic field. This phenomenon is utilized in many applications, such as electron microscopes and mass spectrometers.
The interaction of electrons with electric and magnetic fields is a cornerstone of many technologies, including television screens, particle accelerators, and medical imaging devices.
Electrons in Technology and Beyond: Applications and Significance
Electrons are not just abstract concepts confined to textbooks; they are the fundamental building blocks of countless technologies and play a crucial role in our understanding of the universe.
- Electronics: The flow of electrons in electronic devices allows for the processing and transmission of information. Transistors, integrated circuits, and other electronic components rely on the controlled movement of electrons.
- Medical imaging: Techniques like X-rays, CT scans, and PET scans utilize electron interactions with matter to create images of the human body.
- Particle physics: The study of electrons and their interactions with other particles has contributed significantly to our understanding of fundamental forces and the universe's structure.
- Materials science: Understanding electron behavior is crucial in developing new materials with specific properties, such as superconductivity and high-temperature stability.
Conclusion: The Enduring Importance of the Electron
From their discovery in cathode ray tubes to their crucial roles in modern technology and our understanding of the universe, electrons remain a central focus of scientific inquiry. Their properties, behavior, and interactions continue to fascinate and challenge scientists, driving advancements in diverse fields, from electronics and materials science to medicine and astrophysics. As we continue to explore the intricacies of the quantum world, the humble electron will undoubtedly remain a cornerstone of our scientific understanding. Its fundamental role in shaping the structure and behavior of matter ensures its enduring importance in the ongoing quest to unravel the mysteries of the universe.
Latest Posts
Latest Posts
-
Name The 2 Functional Groups In Amino Acids
Apr 10, 2025
-
E Coli On Eosin Methylene Blue
Apr 10, 2025
-
What Happens When An Acid Is Mixed With A Base
Apr 10, 2025
-
Why Is Electric Field Inside A Conductor Zero
Apr 10, 2025
-
What Do Protons And Neutrons Have In Common
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Negatively Charged Particle In The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.